Pyrrole-26-6YSK
IC50 0.11 +/- 0.01 microM Notum with OPTS substrate
General
Type : Pyrrole_Pyrrolidine-derivative,Trifluoro
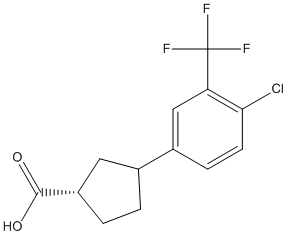
Chemical_Nomenclature : (3S)-1-[4-chloro-3-(trifluoromethyl)phenyl]pyrrolidine-3-carboxylic acid
Canonical SMILES : C1CN(CC1C(=O)O)C2=CC(=C(C=C2)Cl)C(F)(F)F
InChI : InChI=1S\/C12H11ClF3NO2\/c13-10-2-1-8(5-9(10)12(14,15)16)17-4-3-7(6-17)11(18)19\/h1-2,5,7H,3-4,6H2,(H,18,19)\/t7-\/m0\/s1
InChIKey : XTYYFZCQCKEYKG-ZETCQYMHSA-N
Other name(s) : PJK,(3~{S})-1-[4-chloranyl-3-(trifluoromethyl)phenyl]pyrrolidine-3-carboxylic acid
MW : 293.67
Formula : C12H11ClF3NO2
CAS_number :
PubChem : 154585553
UniChem : XTYYFZCQCKEYKG-ZETCQYMHSA-N
IUPHAR :
Wikipedia :
Target
Families : Pyrrole-26-6YSK ligand of proteins in family: Pectinacetylesterase-Notum
Stucture : 6YSK 1-phenylpyrroles and 1-enylpyrrolidines as inhibitors of human Notum
Protein : human-NOTUM
References (1)
| Title : Screening of a Custom-Designed Acid Fragment Library Identifies 1-Phenylpyrroles and 1-Phenylpyrrolidines as Inhibitors of Notum Carboxylesterase Activity - Mahy_2020_J.Med.Chem_63_9464 |
| Author(s) : Mahy W , Patel M , Steadman D , Woodward HL , Atkinson BN , Svensson F , Willis NJ , Flint A , Papatheodorou D , Zhao Y , Vecchia L , Ruza RR , Hillier J , Frew S , Monaghan A , Costa A , Bictash M , Walter MW , Jones EY , Fish PV |
| Ref : Journal of Medicinal Chemistry , 63 :9464 , 2020 |
| Abstract : Mahy_2020_J.Med.Chem_63_9464 |
| ESTHER : Mahy_2020_J.Med.Chem_63_9464 |
| PubMedSearch : Mahy_2020_J.Med.Chem_63_9464 |
| PubMedID: 32787107 |
| Gene_locus related to this paper: human-NOTUM |