Solanacol
General
Type : Strigolactone receptors ligand,Canonical strigolactone
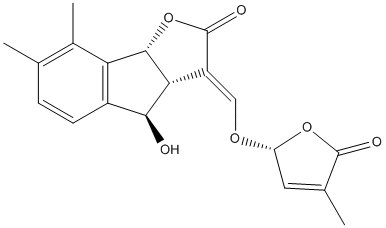
Chemical_Nomenclature : (3E,3aR,4R,8bR)-4-hydroxy-7,8-dimethyl-3-[[(2R)-4-methyl-5-oxo-2H-furan-2-yl]oxymethylidene]-4,8b-dihydro-3aH-indeno[1,2-b]furan-2-one
Canonical SMILES : CC1=C(C2=C(C=C1)C(C3C2OC(=O)C3=COC4C=C(C(=O)O4)C)O)C
InChI : InChI=1S\/C19H18O6\/c1-8-4-5-11-14(10(8)3)17-15(16(11)20)12(19(22)25-17)7-23-13-6-9(2)18(21)24-13\/h4-7,13,15-17,20H,1-3H3\/b12-7+\/t13-,15-,16+,17+\/m1\/s1
InChIKey : LFVSQVHIKFDYFI-ISGZTCCGSA-N
Other name(s) : UNII-8870D88Y8M,8870D88Y8M,(-)-Solanacol,SCHEMBL16203012
MW : 342.3
Formula : C19H18O6
CAS_number : 953389-72-5
PubChem : 102417064, 117672396, 131841610, 51041887
UniChem : LFVSQVHIKFDYFI-ISGZTCCGSA-N
IUPHAR :
Wikipedia :
Target
References (18)
| Title : The tomato cytochrome P450 CYP712G1 catalyses the double oxidation of orobanchol en route to the rhizosphere signalling strigolactone, solanacol - Wang_2022_New.Phytol__ |
| Author(s) : Wang Y , Durairaj J , Suarez Duran HG , van Velzen R , Flokova K , Liao CY , Chojnacka A , MacFarlane S , Schranz ME , Medema MH , van Dijk ADJ , Dong L , Bouwmeester HJ |
| Ref : New Phytol , : , 2022 |
| Abstract : Wang_2022_New.Phytol__ |
| ESTHER : Wang_2022_New.Phytol__ |
| PubMedSearch : Wang_2022_New.Phytol__ |
| PubMedID: 35612785 |
| Title : Noncanonical Strigolactone Analogues Highlight Selectivity for Stimulating Germination in Two Phelipanche ramosa Populations - Fornier_2022_J.Nat.Prod__ |
| Author(s) : Fornier SD , de Saint Germain A , Retailleau P , Pillot JP , Taulera Q , Andna L , Miesch L , Rochange S , Pouvreau JB , Boyer FD |
| Ref : Journal of Natural Products , : , 2022 |
| Abstract : Fornier_2022_J.Nat.Prod__ |
| ESTHER : Fornier_2022_J.Nat.Prod__ |
| PubMedSearch : Fornier_2022_J.Nat.Prod__ |
| PubMedID: 35776904 |
| Title : Phytochemical Study of Safflower Roots (Carthamus tinctorius) on the Induction of Parasitic Plant Germination and Weed Control - Rial_2020_J.Chem.Ecol_46_871 |
| Author(s) : Rial C , Tome S , Varela RM , Molinillo JMG , Macias FA |
| Ref : J Chem Ecol , 46 :871 , 2020 |
| Abstract : Rial_2020_J.Chem.Ecol_46_871 |
| ESTHER : Rial_2020_J.Chem.Ecol_46_871 |
| PubMedSearch : Rial_2020_J.Chem.Ecol_46_871 |
| PubMedID: 32691372 |
| Title : A new UHPLC-MS\/MS method for the direct determination of strigolactones in root exudates and extracts - Rial_2019_Phytochem.Anal_30_110 |
| Author(s) : Rial C , Varela RM , Molinillo JMG , Lopez-Raez JA , Macias FA |
| Ref : Phytochem Anal , 30 :110 , 2019 |
| Abstract : Rial_2019_Phytochem.Anal_30_110 |
| ESTHER : Rial_2019_Phytochem.Anal_30_110 |
| PubMedSearch : Rial_2019_Phytochem.Anal_30_110 |
| PubMedID: 30280444 |
| Title : The tomato MAX1 homolog, SlMAX1, is involved in the biosynthesis of tomato strigolactones from carlactone - Zhang_2018_New.Phytol_219_297 |
| Author(s) : Zhang Y , Cheng X , Wang Y , Diez-Simon C , Flokova K , Bimbo A , Bouwmeester HJ , Ruyter-Spira C |
| Ref : New Phytol , 219 :297 , 2018 |
| Abstract : Zhang_2018_New.Phytol_219_297 |
| ESTHER : Zhang_2018_New.Phytol_219_297 |
| PubMedSearch : Zhang_2018_New.Phytol_219_297 |
| PubMedID: 29655242 |
| Title : Enantioselective total synthesis and biological evaluation of (-)-solanacol - Bromhead_2018_Org.Biomol.Chem_16_5500 |
| Author(s) : Bromhead LJ , Norman AR , Snowden KC , Janssen BJ , McErlean CSP |
| Ref : Org Biomol Chem , 16 :5500 , 2018 |
| Abstract : Bromhead_2018_Org.Biomol.Chem_16_5500 |
| ESTHER : Bromhead_2018_Org.Biomol.Chem_16_5500 |
| PubMedSearch : Bromhead_2018_Org.Biomol.Chem_16_5500 |
| PubMedID: 30027185 |
| Title : Low Infection of Phelipanche aegyptiaca in Micro-Tom Mutants Deficient in CAROTENOIDCLEAVAGE DIOXYGENASE 8 - Hasegawa_2018_Int.J.Mol.Sci_19_ |
| Author(s) : Hasegawa S , Tsutsumi T , Fukushima S , Okabe Y , Saito J , Katayama M , Shindo M , Yamada Y , Shimomura K , Yoneyama K , Akiyama K , Aoki K , Ariizumi T , Ezura H , Yamaguchi S , Umehara M |
| Ref : Int J Mol Sci , 19 : , 2018 |
| Abstract : Hasegawa_2018_Int.J.Mol.Sci_19_ |
| ESTHER : Hasegawa_2018_Int.J.Mol.Sci_19_ |
| PubMedSearch : Hasegawa_2018_Int.J.Mol.Sci_19_ |
| PubMedID: 30200620 |
| Title : A concise synthesis of optically active solanacol, the germination stimulant for seeds of root parasitic weeds - Kumagai_2015_Biosci.Biotechnol.Biochem_79_1240 |
| Author(s) : Kumagai H , Fujiwara M , Kuse M , Takikawa H |
| Ref : Biosci Biotechnol Biochem , 79 :1240 , 2015 |
| Abstract : Kumagai_2015_Biosci.Biotechnol.Biochem_79_1240 |
| ESTHER : Kumagai_2015_Biosci.Biotechnol.Biochem_79_1240 |
| PubMedSearch : Kumagai_2015_Biosci.Biotechnol.Biochem_79_1240 |
| PubMedID: 25798895 |
| Title : Palladium(II)-catalyzed intramolecular carboxypalladation-olefin insertion cascade: direct access to indeno[1,2-b]furan-2-ones - Vinoth_2015_Org.Biomol.Chem_13_5175 |
| Author(s) : Vinoth P , Vivekanand T , Suryavanshi PA , Menendez JC , Sasai H , Sridharan V |
| Ref : Org Biomol Chem , 13 :5175 , 2015 |
| Abstract : Vinoth_2015_Org.Biomol.Chem_13_5175 |
| ESTHER : Vinoth_2015_Org.Biomol.Chem_13_5175 |
| PubMedSearch : Vinoth_2015_Org.Biomol.Chem_13_5175 |
| PubMedID: 25849414 |
| Title : Confirming stereochemical structures of strigolactones produced by rice and tobacco - Xie_2013_Mol.Plant_6_153 |
| Author(s) : Xie X , Yoneyama K , Kisugi T , Uchida K , Ito S , Akiyama K , Hayashi H , Yokota T , Nomura T |
| Ref : Mol Plant , 6 :153 , 2013 |
| Abstract : Xie_2013_Mol.Plant_6_153 |
| ESTHER : Xie_2013_Mol.Plant_6_153 |
| PubMedSearch : Xie_2013_Mol.Plant_6_153 |
| PubMedID: 23204500 |
| Title : Structure and activity of strigolactones: new plant hormones with a rich future - Zwanenburg_2013_Mol.Plant_6_38 |
| Author(s) : Zwanenburg B , Pospisil T |
| Ref : Mol Plant , 6 :38 , 2013 |
| Abstract : Zwanenburg_2013_Mol.Plant_6_38 |
| ESTHER : Zwanenburg_2013_Mol.Plant_6_38 |
| PubMedSearch : Zwanenburg_2013_Mol.Plant_6_38 |
| PubMedID: 23204499 |
| Title : Tomato strigolactones: a more detailed look - Kohlen_2013_Plant.Signal.Behav_8_e22785 |
| Author(s) : Kohlen W , Charnikhova T , Bours R , Lopez-Raez JA , Bouwmeester H |
| Ref : Plant Signal Behav , 8 :e22785 , 2013 |
| Abstract : Kohlen_2013_Plant.Signal.Behav_8_e22785 |
| ESTHER : Kohlen_2013_Plant.Signal.Behav_8_e22785 |
| PubMedSearch : Kohlen_2013_Plant.Signal.Behav_8_e22785 |
| PubMedID: 23221743 |
| Title : Synthetic studies on the solanacol ABC ring system by cation-initiated cascade cyclization: implications for strigolactone biosynthesis - Chojnacka_2011_Org.Biomol.Chem_9_5350 |
| Author(s) : Chojnacka K , Santoro S , Awartani R , Richards NG , Himo F , Aponick A |
| Ref : Org Biomol Chem , 9 :5350 , 2011 |
| Abstract : Chojnacka_2011_Org.Biomol.Chem_9_5350 |
| ESTHER : Chojnacka_2011_Org.Biomol.Chem_9_5350 |
| PubMedSearch : Chojnacka_2011_Org.Biomol.Chem_9_5350 |
| PubMedID: 21706088 |
| Title : Strigolactone deficiency confers resistance in tomato line SL-ORT1 to the parasitic weeds Phelipanche and Orobanche spp - Dor_2011_Phytopathology_101_213 |
| Author(s) : Dor E , Yoneyama K , Wininger S , Kapulnik Y , Koltai H , Xie X , Hershenhorn J |
| Ref : Phytopathology , 101 :213 , 2011 |
| Abstract : Dor_2011_Phytopathology_101_213 |
| ESTHER : Dor_2011_Phytopathology_101_213 |
| PubMedSearch : Dor_2011_Phytopathology_101_213 |
| PubMedID: 20942651 |
| Title : A tomato strigolactone-impaired mutant displays aberrant shoot morphology and plant interactions - Koltai_2010_J.Exp.Bot_61_1739 |
| Author(s) : Koltai H , LekKala SP , Bhattacharya C , Mayzlish-Gati E , Resnick N , Wininger S , Dor E , Yoneyama K , Hershenhorn J , Joel DM , Kapulnik Y |
| Ref : J Exp Bot , 61 :1739 , 2010 |
| Abstract : Koltai_2010_J.Exp.Bot_61_1739 |
| ESTHER : Koltai_2010_J.Exp.Bot_61_1739 |
| PubMedSearch : Koltai_2010_J.Exp.Bot_61_1739 |
| PubMedID: 20194924 |
| Title : Tomato strigolactones are derived from carotenoids and their biosynthesis is promoted by phosphate starvation - Lopez-Raez_2008_New.Phytol_178_863 |
| Author(s) : Lopez-Raez JA , Charnikhova T , Gomez-Roldan V , Matusova R , Kohlen W , De Vos R , Verstappen F , Puech-Pages V , Becard G , Mulder P , Bouwmeester H |
| Ref : New Phytol , 178 :863 , 2008 |
| Abstract : Lopez-Raez_2008_New.Phytol_178_863 |
| ESTHER : Lopez-Raez_2008_New.Phytol_178_863 |
| PubMedSearch : Lopez-Raez_2008_New.Phytol_178_863 |
| PubMedID: 18346111 |
| Title : 2'-epi-orobanchol and solanacol, two unique strigolactones, germination stimulants for root parasitic weeds, produced by tobacco - Xie_2007_J.Agric.Food.Chem_55_8067 |
| Author(s) : Xie X , Kusumoto D , Takeuchi Y , Yoneyama K , Yamada Y |
| Ref : Journal of Agricultural and Food Chemistry , 55 :8067 , 2007 |
| Abstract : Xie_2007_J.Agric.Food.Chem_55_8067 |
| ESTHER : Xie_2007_J.Agric.Food.Chem_55_8067 |
| PubMedSearch : Xie_2007_J.Agric.Food.Chem_55_8067 |
| PubMedID: 17803261 |