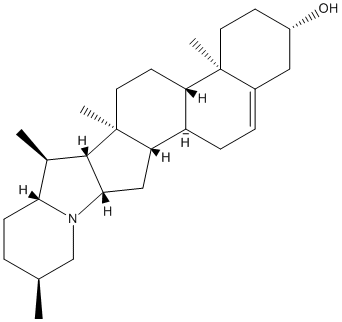
Solanidine
Solanidine is a steroidal alkaloid, and its glycosides have been reported to have caused poisoning in man and animals. Solanidine is present in sera of healthy individuals and in amounts dependent on their dietary potato consumption.
General
Type : Natural,Alkaloid,Steroid
Chemical_Nomenclature : (1S,2S,7S,10R,11S,14S,15R,16S,17R,20S,23S)-10,14,16,20-tetramethyl-22-azahexacyclo[12.10.0.02,11.05,10.015,23.017,22]tetracos-4-en-7-ol
Canonical SMILES : CC1CCC2C(C3C(N2C1)CC4C3(CCC5C4CC=C6C5(CCC(C6)O)C)C)C
InChI : InChI=1S\/C27H43NO\/c1-16-5-8-23-17(2)25-24(28(23)15-16)14-22-20-7-6-18-13-19(29)9-11-26(18,3)21(20)10-12-27(22,25)4\/h6,16-17,19-25,29H,5,7-15H2,1-4H3\/t16-,17+,19-,20+,21-,22-,23+,24-,25-,26-,27-\/m0\/s1
InChIKey : JVKYZPBMZPJNAJ-OQFNDJACSA-N
Other name(s) : Solanidin,Solatubin,Solatubine,22R,25S-Solanidanine,UNII-W7801OHM8B,CCRIS 6508,EINECS 201-309-5,NSC 76025,BRN 0045370,W7801OHM8B,CHEBI:28374,NSC76025,AC1L23U5,CHEMBL1980466,ZINC8220551
MW : 397.64
Formula : C27H43NO
CAS_number : 80-78-4
PubChem :
UniChem : JVKYZPBMZPJNAJ-OQFNDJACSA-N
IUPHAR :
Wikipedia : Solanidine
Target
References (7)
| Title : Structure-based virtual screening leading to discovery of highly selective butyrylcholinesterase inhibitors with solanaceous alkaloid scaffolds - Zhou_2019_Chem.Biol.Interact_13ChEPon_308_372 |
| Author(s) : Zhou S , Yuan Y , Zheng F , Zhan CG |
| Ref : Chemico-Biological Interactions , 308 :372 , 2019 |
| Abstract : Zhou_2019_Chem.Biol.Interact_13ChEPon_308_372 |
| ESTHER : Zhou_2019_Chem.Biol.Interact_13ChEPon_308_372 |
| PubMedSearch : Zhou_2019_Chem.Biol.Interact_13ChEPon_308_372 |
| PubMedID: 31152736 |
| Title : The Impact of Steroidal Glycoalkaloids on the Physiology of Phytophthora infestans, the Causative Agent of Potato Late Blight - Dahlin_2017_Mol.Plant.Microbe.Interact_30_531 |
| Author(s) : Dahlin P , Muller MC , Ekengren S , McKee LS , Bulone V |
| Ref : Mol Plant Microbe Interact , 30 :531 , 2017 |
| Abstract : Dahlin_2017_Mol.Plant.Microbe.Interact_30_531 |
| ESTHER : Dahlin_2017_Mol.Plant.Microbe.Interact_30_531 |
| PubMedSearch : Dahlin_2017_Mol.Plant.Microbe.Interact_30_531 |
| PubMedID: 28510502 |
| Title : A novel enzyme biosensor for steroidal glycoalkaloids detection based on pH-sensitive field effect transistors - Korpan_2002_Bioelectrochemistry_55_9 |
| Author(s) : Korpan YI , Volotovsky VV , Martelet C , Jaffrezic-Renault N , Nazarenko EA , El'skaya AV , Soldatkin AP |
| Ref : Bioelectrochemistry , 55 :9 , 2002 |
| Abstract : Korpan_2002_Bioelectrochemistry_55_9 |
| ESTHER : Korpan_2002_Bioelectrochemistry_55_9 |
| PubMedSearch : Korpan_2002_Bioelectrochemistry_55_9 |
| PubMedID: 11786329 |
| Title : Inhibition of human plasma and serum butyrylcholinesterase (EC 3.1.1.8) by alpha-chaconine and alpha-solanine - Nigg_1996_Fundam.Appl.Toxicol_33_272 |
| Author(s) : Nigg HN , Ramos LE , Graham EM , Sterling J , Brown S , Cornell JA |
| Ref : Fundamental & Applied Toxicology , 33 :272 , 1996 |
| Abstract : Nigg_1996_Fundam.Appl.Toxicol_33_272 |
| ESTHER : Nigg_1996_Fundam.Appl.Toxicol_33_272 |
| PubMedSearch : Nigg_1996_Fundam.Appl.Toxicol_33_272 |
| PubMedID: 8921346 |
| Title : Intramolecular relationships in cholinesterases revealed by oocyte expression of site-directed and natural variants of human BCHE - Neville_1992_EMBO.J_11_1641 |
| Author(s) : Neville LF , Gnatt A , Loewenstein Y , Seidman S , Ehrlich G , Soreq H |
| Ref : EMBO Journal , 11 :1641 , 1992 |
| Abstract : Neville_1992_EMBO.J_11_1641 |
| ESTHER : Neville_1992_EMBO.J_11_1641 |
| PubMedSearch : Neville_1992_EMBO.J_11_1641 |
| PubMedID: 1373381 |
| Title : Aspartate-70 to glycine substitution confers resistance to naturally occurring and synthetic anionic-site ligands on in-ovo produced human butyrylcholinesterase - Neville_1990_J.Neurosci.Res_27_452 |
| Author(s) : Neville LF , Gnatt A , Loewenstein Y , Soreq H |
| Ref : Journal of Neuroscience Research , 27 :452 , 1990 |
| Abstract : Neville_1990_J.Neurosci.Res_27_452 |
| ESTHER : Neville_1990_J.Neurosci.Res_27_452 |
| PubMedSearch : Neville_1990_J.Neurosci.Res_27_452 |
| PubMedID: 2079709 |
| Gene_locus related to this paper: human-BCHE |
| Title : Differential inhibition of the serum cholinesterase phenotypes by solanine and solanidine - |
| Author(s) : Harris H , Whittaker M |
| Ref : Annals of Human Genetics , 26 :73 , 1962 |
| PubMedID: 13904837 |