TPHP
Triphenyl phosphate TPP, Organophosphorus flame retardant. May have adipogenic effects through PPARgamma activation. Triphenyl phosphate permeates the blood brain barrier and induces neurotoxicity in mouse brain. TPP is a widely used synergist, which can reduce the use of pesticides (chemicals or virus) by inhibiting carboxylesterase
General
Type : Organophosphorus flame retardant plasticizer,Organophosphate,Synergist of insecticide
Chemical_Nomenclature :
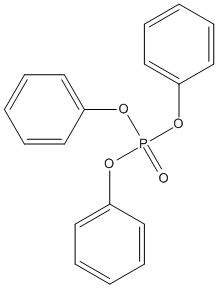
Canonical SMILES : C1=CC=C(C=C1)OP(=O)(OC2=CC=CC=C2)OC3=CC=CC=C3
InChI : InChI=1S\/C18H15O4P\/c19-23(20-16-10-4-1-5-11-16,21-17-12-6-2-7-13-17)22-18-14-8-3-9-15-18\/h1-15H
InChIKey : XZZNDPSIHUTMOC-UHFFFAOYSA-N
Other name(s) : triphenyl phosphate,TPP,Component of Firemaster 550,(C6H5)3PO4,Triphenylphosphate,Phosphoric acid, triphenyl ester,Disflamoll TP
MW : 326.3
Formula : C18H15O4P
CAS_number : 115-86-6
PubChem : 8289
UniChem : XZZNDPSIHUTMOC-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
Families :
Stucture :
Protein :
References (50)
| Title : Organophosphate toxicity patterns: A new approach for assessing organophosphate neurotoxicity - Karaboga_2024_J.Hazard.Mater_470_134236 |
| Author(s) : Karaboga S , Severac F , Collins ES , Stab A , Davis A , Souchet M , Herve G |
| Ref : J Hazard Mater , 470 :134236 , 2024 |
| Abstract : Karaboga_2024_J.Hazard.Mater_470_134236 |
| ESTHER : Karaboga_2024_J.Hazard.Mater_470_134236 |
| PubMedSearch : Karaboga_2024_J.Hazard.Mater_470_134236 |
| PubMedID: 38613959 |
| Title : Associations between acetylcholinesterase-1 mutations and chlorpyrifos resistance in beet armyworm, Spodoptera exigua - Teng_2022_Pestic.Biochem.Physiol_184_105105 |
| Author(s) : Teng H , Zuo Y , Jin Z , Wu Y , Yang Y |
| Ref : Pestic Biochem Physiol , 184 :105105 , 2022 |
| Abstract : Teng_2022_Pestic.Biochem.Physiol_184_105105 |
| ESTHER : Teng_2022_Pestic.Biochem.Physiol_184_105105 |
| PubMedSearch : Teng_2022_Pestic.Biochem.Physiol_184_105105 |
| PubMedID: 35715044 |
| Gene_locus related to this paper: spolt-ACHE1 |
| Title : Linking biochemical and individual-level effects of chlorpyrifos, triphenyl phosphate, and bisphenol A on sea urchin (Paracentrotus lividus) larvae - Bellas_2022_Environ.Sci.Pollut.Res.Int__ |
| Author(s) : Bellas J , Rial D , Valdes J , Vidal-Linan L , Bertucci JI , Muniategui S , Leon VM , Campillo JA |
| Ref : Environ Sci Pollut Res Int , : , 2022 |
| Abstract : Bellas_2022_Environ.Sci.Pollut.Res.Int__ |
| ESTHER : Bellas_2022_Environ.Sci.Pollut.Res.Int__ |
| PubMedSearch : Bellas_2022_Environ.Sci.Pollut.Res.Int__ |
| PubMedID: 35165844 |
| Title : Contribution of multiple overexpressed carboxylesterase genes to indoxacarb resistance in Spodoptera litura - Shi_2022_Pest.Manag.Sci__ |
| Author(s) : Shi Y , Li W , Zhou Y , Liao X , Shi L |
| Ref : Pest Manag Sci , : , 2022 |
| Abstract : Shi_2022_Pest.Manag.Sci__ |
| ESTHER : Shi_2022_Pest.Manag.Sci__ |
| PubMedSearch : Shi_2022_Pest.Manag.Sci__ |
| PubMedID: 35066991 |
| Title : Recombinant AcMNPV-gp64-EGFP and synergist triphenyl phosphate, an effective combination against Spodoptera frugiperda - Jiao_2022_Biotechnol.Lett__ |
| Author(s) : Jiao R , Fu Y |
| Ref : Biotechnol Lett , : , 2022 |
| Abstract : Jiao_2022_Biotechnol.Lett__ |
| ESTHER : Jiao_2022_Biotechnol.Lett__ |
| PubMedSearch : Jiao_2022_Biotechnol.Lett__ |
| PubMedID: 35922646 |
| Title : Occurrence and in vitro toxicity of organic compounds in urban background PM(2.5) - Wallraff_2022_Sci.Total.Environ__152779 |
| Author(s) : Wallraff JP , Ungeheuer F , Dombrowski A , Oehlmann J , Vogel AL |
| Ref : Sci Total Environ , :152779 , 2022 |
| Abstract : Wallraff_2022_Sci.Total.Environ__152779 |
| ESTHER : Wallraff_2022_Sci.Total.Environ__152779 |
| PubMedSearch : Wallraff_2022_Sci.Total.Environ__152779 |
| PubMedID: 35007573 |
| Title : Bioremediation of triphenyl phosphate by Pycnoporus sanguineus: Metabolic pathway, proteomic mechanism and biotoxicity assessment - Feng_2021_J.Hazard.Mater_417_125983 |
| Author(s) : Feng M , Zhou J , Yu X , Wang H , Guo Y , Mao W |
| Ref : J Hazard Mater , 417 :125983 , 2021 |
| Abstract : Feng_2021_J.Hazard.Mater_417_125983 |
| ESTHER : Feng_2021_J.Hazard.Mater_417_125983 |
| PubMedSearch : Feng_2021_J.Hazard.Mater_417_125983 |
| PubMedID: 33975170 |
| Title : Two carboxylesterase genes in Plutella xylostella associated with sex pheromones and plant volatiles degradation - Wang_2021_Pest.Manag.Sci__ |
| Author(s) : Wang MM , Long GJ , Guo H , Liu XZ , Wang H , Dewer Y , Li ZQ , Liu K , Zhang QL , Ma YF , He P , He M |
| Ref : Pest Manag Sci , : , 2021 |
| Abstract : Wang_2021_Pest.Manag.Sci__ |
| ESTHER : Wang_2021_Pest.Manag.Sci__ |
| PubMedSearch : Wang_2021_Pest.Manag.Sci__ |
| PubMedID: 33527628 |
| Gene_locus related to this paper: pluxy-CCE016a , pluxy-CCE016c |
| Title : Characterization of the insecticide detoxification carboxylesterase Boest1 from Bradysia odoriphaga Yang et Zhang (Diptera:Sciaridae) - Ding_2021_Pest.Manag.Sci__ |
| Author(s) : Ding Q , Xu X , Sang Z , Wang R , Ullah F , Gao X , Song D |
| Ref : Pest Manag Sci , : , 2021 |
| Abstract : Ding_2021_Pest.Manag.Sci__ |
| ESTHER : Ding_2021_Pest.Manag.Sci__ |
| PubMedSearch : Ding_2021_Pest.Manag.Sci__ |
| PubMedID: 34596943 |
| Gene_locus related to this paper: 9dipt-Boest1 , braco-est1 |
| Title : Triphenyl phosphate disturbs the lipidome and induces endoplasmic reticulum stress and apoptosis in JEG-3 cells - Wang_2021_Chemosphere_275_129978 |
| Author(s) : Wang Y , Hong J , Shi M , Guo L , Liu L , Tang H , Liu X |
| Ref : Chemosphere , 275 :129978 , 2021 |
| Abstract : Wang_2021_Chemosphere_275_129978 |
| ESTHER : Wang_2021_Chemosphere_275_129978 |
| PubMedSearch : Wang_2021_Chemosphere_275_129978 |
| PubMedID: 33662732 |
| Title : In Vitro Metabolism of Isopropylated and tert-Butylated Triarylphosphate Esters Using Human Liver Subcellular Fractions - Phillips_2020_Chem.Res.Toxicol_33_1428 |
| Author(s) : Phillips AL , Herkert NJ , Ulrich JC , Hartman JH , Ruis MT , Cooper EM , Ferguson PL , Stapleton HM |
| Ref : Chemical Research in Toxicology , 33 :1428 , 2020 |
| Abstract : Phillips_2020_Chem.Res.Toxicol_33_1428 |
| ESTHER : Phillips_2020_Chem.Res.Toxicol_33_1428 |
| PubMedSearch : Phillips_2020_Chem.Res.Toxicol_33_1428 |
| PubMedID: 32129605 |
| Title : Organophosphorus flame retardant induced hepatotoxicity and brain AChE inhibition on zebrafish (Danio rerio) - Ramesh_2020_Neurotoxicol.Teratol__106919 |
| Author(s) : Ramesh M , Angitha S , Haritha S , Poopal RK , Ren Z , Umamaheswari S |
| Ref : Neurotoxicology & Teratology , :106919 , 2020 |
| Abstract : Ramesh_2020_Neurotoxicol.Teratol__106919 |
| ESTHER : Ramesh_2020_Neurotoxicol.Teratol__106919 |
| PubMedSearch : Ramesh_2020_Neurotoxicol.Teratol__106919 |
| PubMedID: 32853706 |
| Title : Inhibitory effects of organophosphate esters on carboxylesterase activity of rat liver microsomes - Tsugoshi_2020_Chem.Biol.Interact__109148 |
| Author(s) : Tsugoshi Y , Watanabe Y , Tanikawa Y , Inoue C , Sugihara K , Kojima H , Kitamura S |
| Ref : Chemico-Biological Interactions , :109148 , 2020 |
| Abstract : Tsugoshi_2020_Chem.Biol.Interact__109148 |
| ESTHER : Tsugoshi_2020_Chem.Biol.Interact__109148 |
| PubMedSearch : Tsugoshi_2020_Chem.Biol.Interact__109148 |
| PubMedID: 32511959 |
| Title : In vitro biolayer interferometry analysis of acetylcholinesterase as a potential target of aryl-organophosphorus flame-retardants - Shi_2020_J.Hazard.Mater_409_124999 |
| Author(s) : Shi Q , Guo W , Shen Q , Han J , Lei L , Chen L , Yang L , Feng C , Zhou B |
| Ref : J Hazard Mater , 409 :124999 , 2020 |
| Abstract : Shi_2020_J.Hazard.Mater_409_124999 |
| ESTHER : Shi_2020_J.Hazard.Mater_409_124999 |
| PubMedSearch : Shi_2020_J.Hazard.Mater_409_124999 |
| PubMedID: 33454525 |
| Title : Susceptibility of fall armyworm, Spodoptera frugiperda (J.E.Smmith), to eight insecticides in China, with special reference to lambda-cyhalothrin - Zhao_2020_Pestic.Biochem.Physiol_168_104623 |
| Author(s) : Zhao YX , Huang JM , Ni H , Guo D , Yang FX , Wang X , Wu SF , Gao CF |
| Ref : Pestic Biochem Physiol , 168 :104623 , 2020 |
| Abstract : Zhao_2020_Pestic.Biochem.Physiol_168_104623 |
| ESTHER : Zhao_2020_Pestic.Biochem.Physiol_168_104623 |
| PubMedSearch : Zhao_2020_Pestic.Biochem.Physiol_168_104623 |
| PubMedID: 32711763 |
| Title : Cholinesterase Inhibition and Exposure to Organophosphate Esters in Aircraft Maintenance Workers - Hardos_2020_Aerosp.Med.Hum.Perform_91_710 |
| Author(s) : Hardos JE , Rubenstein M , Pfahler S , Sleight T |
| Ref : Aerospace Medicine Hum Perform , 91 :710 , 2020 |
| Abstract : Hardos_2020_Aerosp.Med.Hum.Perform_91_710 |
| ESTHER : Hardos_2020_Aerosp.Med.Hum.Perform_91_710 |
| PubMedSearch : Hardos_2020_Aerosp.Med.Hum.Perform_91_710 |
| PubMedID: 32867901 |
| Title : Adipogenic activity of 2-ethylhexyl diphenyl phosphate via peroxisome proliferator-activated receptor gamma pathway - Sun_2020_Sci.Total.Environ_711_134810 |
| Author(s) : Sun W , Duan X , Chen H , Zhang L , Sun H |
| Ref : Sci Total Environ , 711 :134810 , 2020 |
| Abstract : Sun_2020_Sci.Total.Environ_711_134810 |
| ESTHER : Sun_2020_Sci.Total.Environ_711_134810 |
| PubMedSearch : Sun_2020_Sci.Total.Environ_711_134810 |
| PubMedID: 31812418 |
| Title : Sex- and age-dependent effects of maternal organophosphate flame-retardant exposure on neonatal hypothalamic and hepatic gene expression - Adams_2020_Reprod.Toxicol_94_65 |
| Author(s) : Adams S , Wiersielis K , Yasrebi A , Conde K , Armstrong L , Guo GL , Roepke TA |
| Ref : Reprod Toxicol , 94 :65 , 2020 |
| Abstract : Adams_2020_Reprod.Toxicol_94_65 |
| ESTHER : Adams_2020_Reprod.Toxicol_94_65 |
| PubMedSearch : Adams_2020_Reprod.Toxicol_94_65 |
| PubMedID: 32360330 |
| Title : Triphenyl phosphate permeates the blood brain barrier and induces neurotoxicity in mouse brain - Liu_2020_Chemosphere_252_126470 |
| Author(s) : Liu X , Zhao X , Wang Y , Hong J , Shi M , Pfaff D , Guo L , Tang H |
| Ref : Chemosphere , 252 :126470 , 2020 |
| Abstract : Liu_2020_Chemosphere_252_126470 |
| ESTHER : Liu_2020_Chemosphere_252_126470 |
| PubMedSearch : Liu_2020_Chemosphere_252_126470 |
| PubMedID: 32443258 |
| Title : Inhibition of Human Liver Carboxylesterase (hCE1) by Organophosphate Ester Flame Retardants and Plasticizers: Implications for Pharmacotherapy - Phillips_2019_Toxicol.Sci_171_396 |
| Author(s) : Phillips AL , Stapleton HM |
| Ref : Toxicol Sci , 171 :396 , 2019 |
| Abstract : Phillips_2019_Toxicol.Sci_171_396 |
| ESTHER : Phillips_2019_Toxicol.Sci_171_396 |
| PubMedSearch : Phillips_2019_Toxicol.Sci_171_396 |
| PubMedID: 31268531 |
| Title : Acute exposure to triphenyl phosphate (TPhP) disturbs ocular development and muscular organization in zebrafish larvae - Shi_2019_Ecotoxicol.Environ.Saf_179_119 |
| Author(s) : Shi Q , Tsui MMP , Hu C , Lam JCW , Zhou B , Chen L |
| Ref : Ecotoxicology & Environmental Safety , 179 :119 , 2019 |
| Abstract : Shi_2019_Ecotoxicol.Environ.Saf_179_119 |
| ESTHER : Shi_2019_Ecotoxicol.Environ.Saf_179_119 |
| PubMedSearch : Shi_2019_Ecotoxicol.Environ.Saf_179_119 |
| PubMedID: 31035246 |
| Title : Effect of Synergists on Deltamethrin Resistance in the Common Bed Bug (Hemiptera: Cimicidae) - Gonzalez-Morales_2019_J.Econ.Entomol_112_786 |
| Author(s) : Gonzalez-Morales MA , Romero A |
| Ref : J Econ Entomol , 112 :786 , 2019 |
| Abstract : Gonzalez-Morales_2019_J.Econ.Entomol_112_786 |
| ESTHER : Gonzalez-Morales_2019_J.Econ.Entomol_112_786 |
| PubMedSearch : Gonzalez-Morales_2019_J.Econ.Entomol_112_786 |
| PubMedID: 30535372 |
| Title : Developmental neurotoxicity of triphenyl phosphate in zebrafish larvae - Shi_2018_Aquat.Toxicol_203_80 |
| Author(s) : Shi Q , Wang M , Shi F , Yang L , Guo Y , Feng C , Liu J , Zhou B |
| Ref : Aquat Toxicol , 203 :80 , 2018 |
| Abstract : Shi_2018_Aquat.Toxicol_203_80 |
| ESTHER : Shi_2018_Aquat.Toxicol_203_80 |
| PubMedSearch : Shi_2018_Aquat.Toxicol_203_80 |
| PubMedID: 30096480 |
| Title : Di-branched triphenylamine dye sensitized TiO2 nanocomposites with good photo-stability for sensitive photoelectrochemical detection of organophosphate pesticides - Song_2018_Anal.Chim.Acta_1001_24 |
| Author(s) : Song J , Wu S , Xing P , Zhao Y , Yuan J |
| Ref : Anal Chim Acta , 1001 :24 , 2018 |
| Abstract : Song_2018_Anal.Chim.Acta_1001_24 |
| ESTHER : Song_2018_Anal.Chim.Acta_1001_24 |
| PubMedSearch : Song_2018_Anal.Chim.Acta_1001_24 |
| PubMedID: 29291803 |
| Title : Characterization and functional analysis of a carboxylesterase gene associated with chlorpyrifos resistance in Nilaparvata lugens (Stal) - Lu_2017_Comp.Biochem.Physiol.C.Toxicol.Pharmacol_203_12 |
| Author(s) : Lu K , Wang Y , Chen X , Zhang Z , Li Y , Li W , Zhou Q |
| Ref : Comparative Biochemistry & Physiology C Toxicol Pharmacol , 203 :12 , 2017 |
| Abstract : Lu_2017_Comp.Biochem.Physiol.C.Toxicol.Pharmacol_203_12 |
| ESTHER : Lu_2017_Comp.Biochem.Physiol.C.Toxicol.Pharmacol_203_12 |
| PubMedSearch : Lu_2017_Comp.Biochem.Physiol.C.Toxicol.Pharmacol_203_12 |
| PubMedID: 29054582 |
| Gene_locus related to this paper: nillu-NlCarE |
| Title : Carboxylesterase-involved metabolism of di-n-butyl phthalate in pumpkin (Cucurbita moschata) seedlings - Lin_2017_Environ.Pollut_220_421 |
| Author(s) : Lin Q , Chen S , Chao Y , Huang X , Wang S , Qiu R |
| Ref : Environ Pollut , 220 :421 , 2017 |
| Abstract : Lin_2017_Environ.Pollut_220_421 |
| ESTHER : Lin_2017_Environ.Pollut_220_421 |
| PubMedSearch : Lin_2017_Environ.Pollut_220_421 |
| PubMedID: 27697378 |
| Title : Adipogenic Effects and Gene Expression Profiling of Firemaster 550 Components in Human Primary Preadipocytes - Tung_2017_Environ.Health.Perspect_125_097013 |
| Author(s) : Tung EWY , Peshdary V , Gagne R , Rowan-Carroll A , Yauk CL , Boudreau A , Atlas E |
| Ref : Environmental Health Perspectives , 125 :097013 , 2017 |
| Abstract : Tung_2017_Environ.Health.Perspect_125_097013 |
| ESTHER : Tung_2017_Environ.Health.Perspect_125_097013 |
| PubMedSearch : Tung_2017_Environ.Health.Perspect_125_097013 |
| PubMedID: 28934090 |
| Title : Firemaster 550 and its components isopropylated triphenyl phosphate and triphenyl phosphate enhance adipogenesis and transcriptional activity of peroxisome proliferator activated receptor (Ppargamma) on the adipocyte protein 2 (aP2) promoter - Tung_2017_PLoS.One_12_e0175855 |
| Author(s) : Tung EWY , Ahmed S , Peshdary V , Atlas E |
| Ref : PLoS ONE , 12 :e0175855 , 2017 |
| Abstract : Tung_2017_PLoS.One_12_e0175855 |
| ESTHER : Tung_2017_PLoS.One_12_e0175855 |
| PubMedSearch : Tung_2017_PLoS.One_12_e0175855 |
| PubMedID: 28437481 |
| Title : Functional characterization of an alpha-esterase gene involving malathion detoxification in Bactrocera dorsalis (Hendel) - Wang_2016_Pestic.Biochem.Physiol_130_44 |
| Author(s) : Wang LL , Lu XP , Meng LW , Huang Y , Wei D , Jiang HB , Smagghe G , Wang JJ |
| Ref : Pestic Biochem Physiol , 130 :44 , 2016 |
| Abstract : Wang_2016_Pestic.Biochem.Physiol_130_44 |
| ESTHER : Wang_2016_Pestic.Biochem.Physiol_130_44 |
| PubMedSearch : Wang_2016_Pestic.Biochem.Physiol_130_44 |
| PubMedID: 27155483 |
| Gene_locus related to this paper: bacdo-a0a034w7m1 |
| Title : Developmental neurotoxicity of organophosphate flame retardants in early life stages of Japanese medaka (Oryzias latipes) - Sun_2016_Environ.Toxicol.Chem_35_2931 |
| Author(s) : Sun L , Tan H , Peng T , Wang S , Xu W , Qian H , Jin Y , Fu Z |
| Ref : Environ Toxicol Chem , 35 :2931 , 2016 |
| Abstract : Sun_2016_Environ.Toxicol.Chem_35_2931 |
| ESTHER : Sun_2016_Environ.Toxicol.Chem_35_2931 |
| PubMedSearch : Sun_2016_Environ.Toxicol.Chem_35_2931 |
| PubMedID: 27146889 |
| Title : Thiamethoxam resistance selected in the western flower thrips Frankliniella occidentalis (Thysanoptera: Thripidae): Cross-resistance patterns, possible biochemical mechanisms and fitness costs analysis - Gao_2014_Pestic.Biochem.Physiol_114_90 |
| Author(s) : Gao CF , Ma SZ , Shan CH , Wu SF |
| Ref : Pestic Biochem Physiol , 114 :90 , 2014 |
| Abstract : Gao_2014_Pestic.Biochem.Physiol_114_90 |
| ESTHER : Gao_2014_Pestic.Biochem.Physiol_114_90 |
| PubMedSearch : Gao_2014_Pestic.Biochem.Physiol_114_90 |
| PubMedID: 25175655 |
| Title : Esterases of Varroa destructor (Acari: Varroidae), parasitic mite of the honeybee - Dmitryjuk_2014_Exp.Appl.Acarol_62_499 |
| Author(s) : Dmitryjuk M , Zoltowska K , Fraczek R , Lipinski Z |
| Ref : Exp Appl Acarol , 62 :499 , 2014 |
| Abstract : Dmitryjuk_2014_Exp.Appl.Acarol_62_499 |
| ESTHER : Dmitryjuk_2014_Exp.Appl.Acarol_62_499 |
| PubMedSearch : Dmitryjuk_2014_Exp.Appl.Acarol_62_499 |
| PubMedID: 24233156 |
| Title : The involvement of several enzymes in methanol detoxification in Drosophila melanogaster adults - Wang_2013_Comp.Biochem.Physiol.B.Biochem.Mol.Biol_166_7 |
| Author(s) : Wang SP , Hu XX , Meng QW , Muhammad SA , Chen RR , Li F , Li GQ |
| Ref : Comparative Biochemistry & Physiology B Biochem Mol Biol , 166 :7 , 2013 |
| Abstract : Wang_2013_Comp.Biochem.Physiol.B.Biochem.Mol.Biol_166_7 |
| ESTHER : Wang_2013_Comp.Biochem.Physiol.B.Biochem.Mol.Biol_166_7 |
| PubMedSearch : Wang_2013_Comp.Biochem.Physiol.B.Biochem.Mol.Biol_166_7 |
| PubMedID: 23751173 |
| Title : Cross-resistance of bisultap resistant strain of Nilaparvata lugens and its biochemical mechanism - Ling_2011_J.Econ.Entomol_104_243 |
| Author(s) : Ling S , Zhang R |
| Ref : J Econ Entomol , 104 :243 , 2011 |
| Abstract : Ling_2011_J.Econ.Entomol_104_243 |
| ESTHER : Ling_2011_J.Econ.Entomol_104_243 |
| PubMedSearch : Ling_2011_J.Econ.Entomol_104_243 |
| PubMedID: 21404864 |
| Title : Cross-resistance and possible mechanisms of chlorpyrifos resistance in Laodelphax striatellus (Fallen) - Wang_2010_Pest.Manag.Sci_66_1096 |
| Author(s) : Wang L , Zhang Y , Han Z , Liu Y , Fang J |
| Ref : Pest Manag Sci , 66 :1096 , 2010 |
| Abstract : Wang_2010_Pest.Manag.Sci_66_1096 |
| ESTHER : Wang_2010_Pest.Manag.Sci_66_1096 |
| PubMedSearch : Wang_2010_Pest.Manag.Sci_66_1096 |
| PubMedID: 20582994 |
| Title : Mechanisms of organophosphate resistance in a field population of oriental migratory locust, Locusta migratoria manilensis (Meyen) - Yang_2009_Arch.Insect.Biochem.Physiol_71_3 |
| Author(s) : Yang ML , Zhang JZ , Zhu KY , Xuan T , Liu XJ , Guo YP , Ma EB |
| Ref : Archives of Insect Biochemistry & Physiology , 71 :3 , 2009 |
| Abstract : Yang_2009_Arch.Insect.Biochem.Physiol_71_3 |
| ESTHER : Yang_2009_Arch.Insect.Biochem.Physiol_71_3 |
| PubMedSearch : Yang_2009_Arch.Insect.Biochem.Physiol_71_3 |
| PubMedID: 18615705 |
| Title : Differential response to diazinon and coumaphos in a strain of Boophilus microplus (Acari: Ixodidae) collected in Mexico - Miller_2008_J.Med.Entomol_45_905 |
| Author(s) : Miller RJ , Li AY , Tijerina M , Davey RB , George JE |
| Ref : Journal of Medical Entomology , 45 :905 , 2008 |
| Abstract : Miller_2008_J.Med.Entomol_45_905 |
| ESTHER : Miller_2008_J.Med.Entomol_45_905 |
| PubMedSearch : Miller_2008_J.Med.Entomol_45_905 |
| PubMedID: 18826034 |
| Title : Development of a microporous membrane liquid-liquid extractor for organophosphate esters in human blood plasma: identification of triphenyl phosphate and octyl diphenyl phosphate in donor plasma - Jonsson_2001_J.Chromatogr.B.Biomed.Sci.Appl_755_157 |
| Author(s) : Jonsson OB , Dyremark E , Nilsson UL |
| Ref : Journal of Chromatography B Biomed Sci Appl , 755 :157 , 2001 |
| Abstract : Jonsson_2001_J.Chromatogr.B.Biomed.Sci.Appl_755_157 |
| ESTHER : Jonsson_2001_J.Chromatogr.B.Biomed.Sci.Appl_755_157 |
| PubMedSearch : Jonsson_2001_J.Chromatogr.B.Biomed.Sci.Appl_755_157 |
| PubMedID: 11393700 |
| Title : Correlation between biochemical parameters and susceptibility of freshwater fish to malathion - Li_1996_J.Toxicol.Env.Health_48_413 |
| Author(s) : Li S-N , Fan D-F |
| Ref : Journal of Toxicology & Environmental Health , 48 :413 , 1996 |
| Abstract : Li_1996_J.Toxicol.Env.Health_48_413 |
| ESTHER : Li_1996_J.Toxicol.Env.Health_48_413 |
| PubMedSearch : Li_1996_J.Toxicol.Env.Health_48_413 |
| PubMedID: 8691510 |
| Title : Isolation of an esterase conferring insecticide resistance in the mosquito Culex tarsalis - Whyard_1994_Insect.Biochem.Mol.Biol_24_819 |
| Author(s) : Whyard S , Downe AE , Walker VK |
| Ref : Insect Biochemistry & Molecular Biology , 24 :819 , 1994 |
| Abstract : Whyard_1994_Insect.Biochem.Mol.Biol_24_819 |
| ESTHER : Whyard_1994_Insect.Biochem.Mol.Biol_24_819 |
| PubMedSearch : Whyard_1994_Insect.Biochem.Mol.Biol_24_819 |
| PubMedID: 7981729 |
| Title : Insecticide resistance and malathion carboxylesterase in the sheep blowfly, Lucilia cuprina - Whyard_1994_Biochem.Genet_32_9 |
| Author(s) : Whyard S , Russell RJ , Walker VK |
| Ref : Biochemical Genetics , 32 :9 , 1994 |
| Abstract : Whyard_1994_Biochem.Genet_32_9 |
| ESTHER : Whyard_1994_Biochem.Genet_32_9 |
| PubMedSearch : Whyard_1994_Biochem.Genet_32_9 |
| PubMedID: 8031296 |
| Title : Pathogenesis of cholesteryl lipidosis of adrenocortical and ovarian interstitial cells in F344 rats caused by tricresyl phosphate and butylated triphenyl phosphate - Latendresse_1993_Toxicol.Appl.Pharmacol_122_281 |
| Author(s) : Latendresse JR , Azhar S , Brooks CL , Capen CC |
| Ref : Toxicol Appl Pharmacol , 122 :281 , 1993 |
| Abstract : Latendresse_1993_Toxicol.Appl.Pharmacol_122_281 |
| ESTHER : Latendresse_1993_Toxicol.Appl.Pharmacol_122_281 |
| PubMedSearch : Latendresse_1993_Toxicol.Appl.Pharmacol_122_281 |
| PubMedID: 8212010 |
| Title : Structural requirements for the inhibition of human monocyte carboxylesterase by organophosphorus compounds - Saboori_1991_Chem.Biol.Interact_80_327 |
| Author(s) : Saboori AM , Lang DM , Newcombe DS |
| Ref : Chemico-Biological Interactions , 80 :327 , 1991 |
| Abstract : Saboori_1991_Chem.Biol.Interact_80_327 |
| ESTHER : Saboori_1991_Chem.Biol.Interact_80_327 |
| PubMedSearch : Saboori_1991_Chem.Biol.Interact_80_327 |
| PubMedID: 1954660 |
| Title : Action of organophosphates on GABAA receptor and voltage-dependent chloride channels - Gant_1987_Fundam.Appl.Toxicol_9_698 |
| Author(s) : Gant DB , Eldefrawi ME , Eldefrawi AT |
| Ref : Fundamental & Applied Toxicology , 9 :698 , 1987 |
| Abstract : Gant_1987_Fundam.Appl.Toxicol_9_698 |
| ESTHER : Gant_1987_Fundam.Appl.Toxicol_9_698 |
| PubMedSearch : Gant_1987_Fundam.Appl.Toxicol_9_698 |
| PubMedID: 2446940 |
| Title : Phthalates and organophosphorus compounds as cholinesterase inhibitors in fractions of industrial hexane impurities - Vicedo_1985_Arch.Toxicol_57_46 |
| Author(s) : Vicedo JL , Pellin M , Vilanova E |
| Ref : Archives of Toxicology , 57 :46 , 1985 |
| Abstract : Vicedo_1985_Arch.Toxicol_57_46 |
| ESTHER : Vicedo_1985_Arch.Toxicol_57_46 |
| PubMedSearch : Vicedo_1985_Arch.Toxicol_57_46 |
| PubMedID: 4015398 |
| Title : Assessment of the delayed neurotoxic potential of isopropyl triphenylphosphate using a nontraditional testing strategy - Sprague_1984_J.Toxicol.Environ.Health_14_773 |
| Author(s) : Sprague GL , Castles TR , Bickford AA |
| Ref : J Toxicol Environ Health , 14 :773 , 1984 |
| Abstract : Sprague_1984_J.Toxicol.Environ.Health_14_773 |
| ESTHER : Sprague_1984_J.Toxicol.Environ.Health_14_773 |
| PubMedSearch : Sprague_1984_J.Toxicol.Environ.Health_14_773 |
| PubMedID: 6520887 |
| Title : Resistance to organophosphate and carbamate insecticides in Anopheles atroparvus - Hemingway_1983_Parassitologia_25_1 |
| Author(s) : Hemingway J , Davidson G |
| Ref : Parassitologia , 25 :1 , 1983 |
| Abstract : Hemingway_1983_Parassitologia_25_1 |
| ESTHER : Hemingway_1983_Parassitologia_25_1 |
| PubMedSearch : Hemingway_1983_Parassitologia_25_1 |
| PubMedID: 6543929 |
| Title : Biochemical studies on malathion resistance in Anopheles arabiensis from Sudan - Hemingway_1983_Trans.R.Soc.Trop.Med.Hyg_77_477 |
| Author(s) : Hemingway J |
| Ref : Trans R Soc Trop Med Hyg , 77 :477 , 1983 |
| Abstract : Hemingway_1983_Trans.R.Soc.Trop.Med.Hyg_77_477 |
| ESTHER : Hemingway_1983_Trans.R.Soc.Trop.Med.Hyg_77_477 |
| PubMedSearch : Hemingway_1983_Trans.R.Soc.Trop.Med.Hyg_77_477 |
| PubMedID: 6636275 |
| Title : The nature of malathion resistance in a population of Anopheles culicifacies Giles - Herath_1981_Bull.World.Health.Organ_59_383 |
| Author(s) : Herath PR , Davidson G |
| Ref : Bulletin of the World Health Organization , 59 :383 , 1981 |
| Abstract : Herath_1981_Bull.World.Health.Organ_59_383 |
| ESTHER : Herath_1981_Bull.World.Health.Organ_59_383 |
| PubMedSearch : Herath_1981_Bull.World.Health.Organ_59_383 |
| PubMedID: 6976845 |
| Title : Evaluation of cytotoxic responses caused by selected organophosphorus esters in chick sympathetic ganglia cultures - Obersteiner_1978_Can.J.Comp.Med_42_80 |
| Author(s) : Obersteiner EJ , Sharma RP |
| Ref : Can J Comp Med , 42 :80 , 1978 |
| Abstract : Obersteiner_1978_Can.J.Comp.Med_42_80 |
| ESTHER : Obersteiner_1978_Can.J.Comp.Med_42_80 |
| PubMedSearch : Obersteiner_1978_Can.J.Comp.Med_42_80 |
| PubMedID: 565668 |