Tacrine-Melatonin-Feruloyl-5c
Permeable agent with antioxidant properties,cholinesterase inhibitory activity, less hepatotoxicity than tacrine, and neuroprotective capacity, able to activate the Nrf2 transcriptional pathway
General
Type : Derivative of Tacrine,Derivative of Tryptamine,Indole,Multitarget,Feruloyl,Acrylate,Antioxidant,Hydroxycinnamic-ester,Alkyl linked bis-ligand
Chemical_Nomenclature :
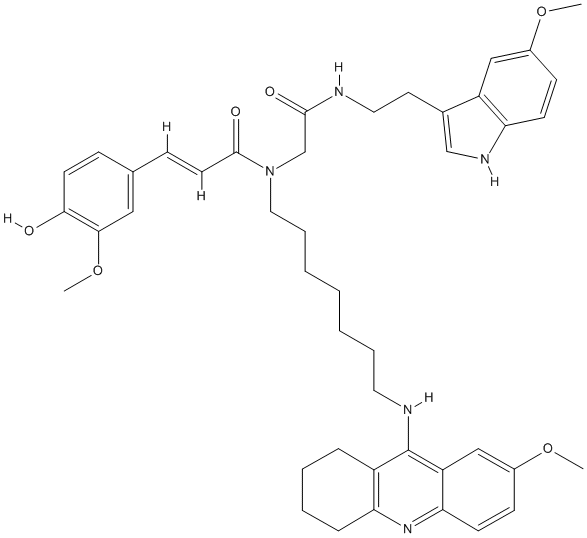
Canonical SMILES : O=C(C(=C(C1=CC(=C(O)C=C1)OC)[H])[H])N(CCCCCCCNC3=C2C=C(OC)C=CC2=NC4=C3CCCC4)CC(NCCC5=C[N]C6=C5C=C(OC)C=C6)=O
InChI : InChI=1S\/C44H53N5O6\/c1-53-32-15-17-37-35(26-32)31(28-47-37)21-23-45-42(51)29-49(43(52)20-14-30-13-19-40(50)41(25-30)55-3)24-10-6-4-5-9-22-46-44-34-11-7-8-12-38(34)48-39-18-16-33(54-2)27-36(39)44\/h13-20,25-28,47,50H,4-12,21-24,29H2,1-3H3,(H,45,51)(H,46,48)\/b20-14+
InChIKey : BCHYGDBEGKZQHD-XSFVSMFZSA-N
Other name(s) :
MW : 747.93
Formula : C44H53N5O6
CAS_number :
PubChem :
UniChem : BCHYGDBEGKZQHD-XSFVSMFZSA-N
IUPHAR :
Wikipedia :
Target
References (1)
| Title : The Antioxidant Additive Approach for Alzheimer's Disease Therapy: New Ferulic (Lipoic) Acid Plus Melatonin Modified Tacrines as Cholinesterases Inhibitors, Direct Antioxidants, and Nuclear Factor (Erythroid-Derived 2)-Like 2 Activators - Benchekroun_2016_J.Med.Chem_59_9967 |
| Author(s) : Benchekroun M , Romero A , Egea J , Leon R , Michalska P , Buendia I , Jimeno ML , Jun D , Janockova J , Sepsova V , Soukup O , Bautista-Aguilera OM , Refouvelet B , Ouari O , Marco-Contelles J , Ismaili L |
| Ref : Journal of Medicinal Chemistry , 59 :9967 , 2016 |
| Abstract : Benchekroun_2016_J.Med.Chem_59_9967 |
| ESTHER : Benchekroun_2016_J.Med.Chem_59_9967 |
| PubMedSearch : Benchekroun_2016_J.Med.Chem_59_9967 |
| PubMedID: 27736061 |