Talinolol
Unselective antagonist of the beta adrenergic receptors (betaAR). Inhibitor of soluble epoxide hydrolase IC50 2.8+/-0.2 muM
General
Type : Urea derivative,Not A\/B H target,Adrenergic-Receptor-ligand,tert-Butyl
Chemical_Nomenclature : 1-[4-[3-(~{tert}-butylamino)-2-hydroxypropoxy]phenyl]-3-cyclohexylurea
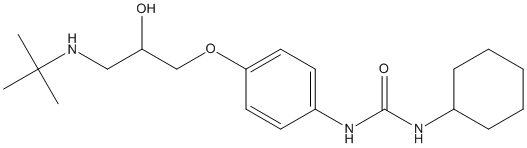
Canonical SMILES : CC(C)(C)NCC(COC1=CC=C(C=C1)NC(=O)NC2CCCCC2)O
InChI : InChI=1S\/C20H33N3O3\/c1-20(2,3)21-13-17(24)14-26-18-11-9-16(10-12-18)23-19(25)22-15-7-5-4-6-8-15\/h9-12,15,17,21,24H,4-8,13-14H2,1-3H3,(H2,22,23,25)
InChIKey : MXFWWQICDIZSOA-UHFFFAOYSA-N
Other name(s) : Cordanum,Racemic talinolol
MW : 363.50
Formula : C20H33N3O3
CAS_number : 57460-41-0
PubChem : 68770
UniChem : MXFWWQICDIZSOA-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
Families : Talinolol ligand of proteins in family: Epoxide_hydrolase
Stucture : 6HGV Human soluble epoxide hydrolase in complex with talinolol
Protein : human-EPHX2
References (1)
| Title : Computer-Aided Selective Optimization of Side Activities of Talinolol - Hiesinger_2019_ACS.Med.Chem.Lett_10_899 |
| Author(s) : Hiesinger K , Kramer JS , Achenbach J , Moser D , Weber J , Wittmann SK , Morisseau C , Angioni C , Geisslinger G , Kahnt AS , Kaiser A , Proschak A , Steinhilber D , Pogoryelov D , Wagner K , Hammock BD , Proschak E |
| Ref : ACS Med Chem Lett , 10 :899 , 2019 |
| Abstract : Hiesinger_2019_ACS.Med.Chem.Lett_10_899 |
| ESTHER : Hiesinger_2019_ACS.Med.Chem.Lett_10_899 |
| PubMedSearch : Hiesinger_2019_ACS.Med.Chem.Lett_10_899 |
| PubMedID: 31223445 |
| Gene_locus related to this paper: human-EPHX2 |