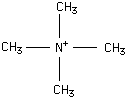
Tetramethylammonium
CID 6380 tetramethylazanium CID 6379 Chloride CID 60966 hydroxyde CID 66137 bromide CID 6381 iodide
General
Type : Quaternary compound,Trimethylammonium
Chemical_Nomenclature : Tetramethylammonium (Iodide)
Canonical SMILES : C[N+](C)(C)C.[Cl-] || C[N+](C)(C)C || C[N+](C)(C)C.[I-] || C[N+](C)(C)C.[OH-] || C[N+](C)(C)C.[Br-]
InChI : InChI=1S\/C4H12N.ClH\/c1-5(2,3)4\;\/h1-4H3\;1H\/q+1\;\/p-1 || InChI=1S\/C4H12N\/c1-5(2,3)4\/h1-4H3\/q+1 || InChI=1S\/C4H12N.HI\/c1-5(2,3)4\;\/h1-4H3\;1H\/q+1\;\/p-1 || InChI=1S\/C4H12N.H2O\/c1-5(2,3)4\;\/h1-4H3\;1H2\/q+1\;\/p-1 || InChI=1S\/C4H12N.BrH\/c1-5(2,3)4\;\/h1-4H3\;1H\/q+1\;\/p-1
InChIKey : OKIZCWYLBDKLSU-UHFFFAOYSA-M || QEMXHQIAXOOASZ-UHFFFAOYSA-N || RXMRGBVLCSYIBO-UHFFFAOYSA-M || WGTYBPLFGIVFAS-UHFFFAOYSA-M || DDFYFBUWEBINLX-UHFFFAOYSA-M
Other name(s) :
Target
References (26)
| Title : The dynamics of ligand barrier crossing inside the acetylcholinesterase gorge - Bui_2003_Biophys.J_85_2267 |
| Author(s) : Bui JM , Henchman RH , McCammon JA |
| Ref : Biophysical Journal , 85 :2267 , 2003 |
| Abstract : Bui_2003_Biophys.J_85_2267 |
| ESTHER : Bui_2003_Biophys.J_85_2267 |
| PubMedSearch : Bui_2003_Biophys.J_85_2267 |
| PubMedID: 14507691 |
| Title : Catalytic antibodies with acetylcholinesterase activity - Johnson_2002_J.Immunol.Methods_269_13 |
| Author(s) : Johnson G , Moore SW |
| Ref : Journal of Immunological Methodsods , 269 :13 , 2002 |
| Abstract : Johnson_2002_J.Immunol.Methods_269_13 |
| ESTHER : Johnson_2002_J.Immunol.Methods_269_13 |
| PubMedSearch : Johnson_2002_J.Immunol.Methods_269_13 |
| PubMedID: 12379349 |
| Title : Idiotypic mimicry of a catalytic antibody active site - Johnson_2002_Mol.Immunol_39_273 |
| Author(s) : Johnson G , Moore SW |
| Ref : Mol Immunol , 39 :273 , 2002 |
| Abstract : Johnson_2002_Mol.Immunol_39_273 |
| ESTHER : Johnson_2002_Mol.Immunol_39_273 |
| PubMedSearch : Johnson_2002_Mol.Immunol_39_273 |
| PubMedID: 12220886 |
| Title : An acetylcholinesterase\/choline oxidase-based amperometric biosensors as a liquid chromatography detector for acetylcholine and choline determination in brain tissue homogenates - Guerrieri_2001_Anal.Chem_73_2875 |
| Author(s) : Guerrieri A , Palmisano F |
| Ref : Analytical Chemistry , 73 :2875 , 2001 |
| Abstract : Guerrieri_2001_Anal.Chem_73_2875 |
| ESTHER : Guerrieri_2001_Anal.Chem_73_2875 |
| PubMedSearch : Guerrieri_2001_Anal.Chem_73_2875 |
| PubMedID: 11467530 |
| Title : Effects of mutations of active site residues and amino acids interacting with the Omega loop on substrate activation of butyrylcholinesterase - Masson_2001_Biochim.Biophys.Acta_1544_166 |
| Author(s) : Masson P , Xie W , Froment MT , Lockridge O |
| Ref : Biochimica & Biophysica Acta , 1544 :166 , 2001 |
| Abstract : Masson_2001_Biochim.Biophys.Acta_1544_166 |
| ESTHER : Masson_2001_Biochim.Biophys.Acta_1544_166 |
| PubMedSearch : Masson_2001_Biochim.Biophys.Acta_1544_166 |
| PubMedID: 11341926 |
| Gene_locus related to this paper: human-BCHE |
| Title : Cholinesterase-like catalytic antibodies: reaction with substrates and inhibitors - Johnson_2000_Mol.Immunol_37_707 |
| Author(s) : Johnson G , Moore SW |
| Ref : Mol Immunol , 37 :707 , 2000 |
| Abstract : Johnson_2000_Mol.Immunol_37_707 |
| ESTHER : Johnson_2000_Mol.Immunol_37_707 |
| PubMedSearch : Johnson_2000_Mol.Immunol_37_707 |
| PubMedID: 11275256 |
| Title : Pathways of ligand clearance in acetylcholinesterase by multiple copy sampling - Van Belle_2000_J.Mol.Biol_298_705 |
| Author(s) : Van Belle D , De Maria L , Iurcu G , Wodak SJ |
| Ref : Journal of Molecular Biology , 298 :705 , 2000 |
| Abstract : Van Belle_2000_J.Mol.Biol_298_705 |
| ESTHER : Van Belle_2000_J.Mol.Biol_298_705 |
| PubMedSearch : Van Belle_2000_J.Mol.Biol_298_705 |
| PubMedID: 10788331 |
| Title : Effect of tetramethylammonium, choline and edrophonium on insect acetylcholinesterase: test of a kinetic model - Stojan_1999_Chem.Biol.Interact_119-120_137 |
| Author(s) : Stojan J , Marcel V , Fournier D |
| Ref : Chemico-Biological Interactions , 119-120 :137 , 1999 |
| Abstract : Stojan_1999_Chem.Biol.Interact_119-120_137 |
| ESTHER : Stojan_1999_Chem.Biol.Interact_119-120_137 |
| PubMedSearch : Stojan_1999_Chem.Biol.Interact_119-120_137 |
| PubMedID: 10421447 |
| Title : Interaction between the peripheral site residues of human butyrylcholinesterase, D70 and Y332, in binding and hydrolysis of substrates - Masson_1999_Biochim.Biophys.Acta_1433_281 |
| Author(s) : Masson P , Xie W , Froment MT , Levitsky V , Fortier PL , Albaret C , Lockridge O |
| Ref : Biochimica & Biophysica Acta , 1433 :281 , 1999 |
| Abstract : Masson_1999_Biochim.Biophys.Acta_1433_281 |
| ESTHER : Masson_1999_Biochim.Biophys.Acta_1433_281 |
| PubMedSearch : Masson_1999_Biochim.Biophys.Acta_1433_281 |
| PubMedID: 10446378 |
| Title : 6-Coumarin diazonium salt: a specific affinity label of the Torpedo acetylcholinesterase peripheral site - Schalk_1995_Mol.Pharmacol_48_1063 |
| Author(s) : Schalk I , Ehret-Sabatier L , Le Feuvre Y , Bon S , Massoulie J , Goeldner M |
| Ref : Molecular Pharmacology , 48 :1063 , 1995 |
| Abstract : Schalk_1995_Mol.Pharmacol_48_1063 |
| ESTHER : Schalk_1995_Mol.Pharmacol_48_1063 |
| PubMedSearch : Schalk_1995_Mol.Pharmacol_48_1063 |
| PubMedID: 8848006 |
| Title : Detection of human DNA mutations with nonradioactive, allele-specific oligonucleotide probes - Hajra_1992_Pharmacogenet_2_78 |
| Author(s) : Hajra A , Sorenson RC , La Du BN |
| Ref : Pharmacogenetics , 2 :78 , 1992 |
| Abstract : Hajra_1992_Pharmacogenet_2_78 |
| ESTHER : Hajra_1992_Pharmacogenet_2_78 |
| PubMedSearch : Hajra_1992_Pharmacogenet_2_78 |
| PubMedID: 1302045 |
| Title : Separation in a single step by affinity chromatography of cholinesterases differing in subunit number - Andres_1991_Protein.Expr.Purif_2_266 |
| Author(s) : Andres C , el Mourabit M , Mark J , Waksman A |
| Ref : Protein Expr Purif , 2 :266 , 1991 |
| Abstract : Andres_1991_Protein.Expr.Purif_2_266 |
| ESTHER : Andres_1991_Protein.Expr.Purif_2_266 |
| PubMedSearch : Andres_1991_Protein.Expr.Purif_2_266 |
| PubMedID: 1821797 |
| Title : General occurrence of binding to acetylcholinesterase-substrate complex in noncompetitive inhibition and in inhibition by substrate - Cohen_1991_Biochim.Biophys.Acta_1076_112 |
| Author(s) : Cohen SG , Chishti SB , Bell DA , Howard SI , Salih E , Cohen JB |
| Ref : Biochimica & Biophysica Acta , 1076 :112 , 1991 |
| Abstract : Cohen_1991_Biochim.Biophys.Acta_1076_112 |
| ESTHER : Cohen_1991_Biochim.Biophys.Acta_1076_112 |
| PubMedSearch : Cohen_1991_Biochim.Biophys.Acta_1076_112 |
| PubMedID: 1986784 |
| Title : Genetic variants of human serum cholinesterase influence metabolism of the muscle relaxant succinylcholine. - Lockridge_1990_Pharmacol.Ther_47_35 |
| Author(s) : Lockridge O |
| Ref : Pharmacol Ther , 47 :35 , 1990 |
| Abstract : Lockridge_1990_Pharmacol.Ther_47_35 |
| ESTHER : Lockridge_1990_Pharmacol.Ther_47_35 |
| PubMedSearch : Lockridge_1990_Pharmacol.Ther_47_35 |
| PubMedID: 2195556 |
| Gene_locus related to this paper: human-BCHE |
| Title : [The specificity of the interaction of the cholinesterases in Far Eastern squid and certain vertebrates with reversible inhibitors] - Rozengart_1988_Zh.Evol.Biokhim.Fiziol_24_679 |
| Author(s) : Rozengart EV , Viniar TN , Kovalev NN , Khovanskikh AE |
| Ref : Zhurnal Evoliutsionnoi Biokhimii i Fiziologii , 24 :679 , 1988 |
| Abstract : Rozengart_1988_Zh.Evol.Biokhim.Fiziol_24_679 |
| ESTHER : Rozengart_1988_Zh.Evol.Biokhim.Fiziol_24_679 |
| PubMedSearch : Rozengart_1988_Zh.Evol.Biokhim.Fiziol_24_679 |
| PubMedID: 3265246 |
| Title : Effects of some mono- and bisquaternary ammonium compounds on the reactivatability of soman-inhibited human acetylcholinesterase in vitro - Hallek_1988_Biochem.Pharmacol_37_819 |
| Author(s) : Hallek M , Szinicz L |
| Ref : Biochemical Pharmacology , 37 :819 , 1988 |
| Abstract : Hallek_1988_Biochem.Pharmacol_37_819 |
| ESTHER : Hallek_1988_Biochem.Pharmacol_37_819 |
| PubMedSearch : Hallek_1988_Biochem.Pharmacol_37_819 |
| PubMedID: 3345199 |
| Title : Structure-activity relationship of reversible cholinesterase inhibitors including paraquat - Seto_1988_Arch.Toxicol_62_37 |
| Author(s) : Seto Y , Shinohara T |
| Ref : Archives of Toxicology , 62 :37 , 1988 |
| Abstract : Seto_1988_Arch.Toxicol_62_37 |
| ESTHER : Seto_1988_Arch.Toxicol_62_37 |
| PubMedSearch : Seto_1988_Arch.Toxicol_62_37 |
| PubMedID: 3190453 |
| Title : Cytogenetic and embryotoxic effects of bromophos and demethylbromophos - Nehez_1986_Regul.Toxicol.Pharmacol_6_416 |
| Author(s) : Nehez M , Huszta E , Mazzag E , Scheufler H , Fischer GW , Desi I |
| Ref : Regul Toxicol Pharmacol , 6 :416 , 1986 |
| Abstract : Nehez_1986_Regul.Toxicol.Pharmacol_6_416 |
| ESTHER : Nehez_1986_Regul.Toxicol.Pharmacol_6_416 |
| PubMedSearch : Nehez_1986_Regul.Toxicol.Pharmacol_6_416 |
| PubMedID: 3809618 |
| Title : Multiple binding of D-tubocurarine to acetylcholinesterase - Zorko_1986_Biochem.Pharmacol_35_2287 |
| Author(s) : Zorko M , Pavlic MR |
| Ref : Biochemical Pharmacology , 35 :2287 , 1986 |
| Abstract : Zorko_1986_Biochem.Pharmacol_35_2287 |
| ESTHER : Zorko_1986_Biochem.Pharmacol_35_2287 |
| PubMedSearch : Zorko_1986_Biochem.Pharmacol_35_2287 |
| PubMedID: 3729986 |
| Title : [Interaction of spin-labeled methacyne analog with butyrylcholinesterase] - Dorokhov_1985_Biofizika_30_23 |
| Author(s) : Dorokhov KE , Grigorian GL , Kardanov NA , Zhdanov RI , Trifonova SA |
| Ref : Biofizika , 30 :23 , 1985 |
| Abstract : Dorokhov_1985_Biofizika_30_23 |
| ESTHER : Dorokhov_1985_Biofizika_30_23 |
| PubMedSearch : Dorokhov_1985_Biofizika_30_23 |
| PubMedID: 2983779 |
| Title : Selective facilitatory effect of vasoactive intestinal polypeptide (VIP) on muscarinic firing in vesical ganglia of the cat - Kawatani_1985_Brain.Res_336_223 |
| Author(s) : Kawatani M , Rutigliano M , De Groat WC |
| Ref : Brain Research , 336 :223 , 1985 |
| Abstract : Kawatani_1985_Brain.Res_336_223 |
| ESTHER : Kawatani_1985_Brain.Res_336_223 |
| PubMedSearch : Kawatani_1985_Brain.Res_336_223 |
| PubMedID: 3891019 |
| Title : Differential inhibition of plasma cholinesterase variants using the dibutyrate analogue of pancuronium bromide - Whittaker_1981_Hum.Hered_31_242 |
| Author(s) : Whittaker M , Britten JJ |
| Ref : Hum Hered , 31 :242 , 1981 |
| Abstract : Whittaker_1981_Hum.Hered_31_242 |
| ESTHER : Whittaker_1981_Hum.Hered_31_242 |
| PubMedSearch : Whittaker_1981_Hum.Hered_31_242 |
| PubMedID: 7287015 |
| Title : Binding constants for tetramethylammonium ion determined with irreversible inhibitors of acetylcholinesterase - Iverson_1976_Can.J.Biochem_54_918 |
| Author(s) : Iverson F |
| Ref : Canadian Journal of Biochemistry , 54 :918 , 1976 |
| Abstract : Iverson_1976_Can.J.Biochem_54_918 |
| ESTHER : Iverson_1976_Can.J.Biochem_54_918 |
| PubMedSearch : Iverson_1976_Can.J.Biochem_54_918 |
| PubMedID: 990993 |
| Title : Interaction of fluorescence probes with acetylcholinesterase. The site and specificity of propidium binding - Taylor_1975_Biochemistry_14_1989 |
| Author(s) : Taylor P , Lappi S |
| Ref : Biochemistry , 14 :1989 , 1975 |
| Abstract : Taylor_1975_Biochemistry_14_1989 |
| ESTHER : Taylor_1975_Biochemistry_14_1989 |
| PubMedSearch : Taylor_1975_Biochemistry_14_1989 |
| PubMedID: 1125207 |
| Title : Activating effect of tetramethylammonium ions on horse scrum cholinesterase - |
| Author(s) : Brestkin AP , Brik IL |
| Ref : Biokhimiia , 32 :1 , 1967 |
| PubMedID: 5591606 |