Trelagliptin
Almost identical to Alogliptin which is a selective, orally bioavailable, pyrimidinedione-based inhibitor of dipeptidyl peptidase 4 (DPP-4), with hypoglycemic activity. Trelagliptin is a fluride derivative.
General
Type : Drug,Piperidine,Pyrimidine,Cyanide,Gliptin
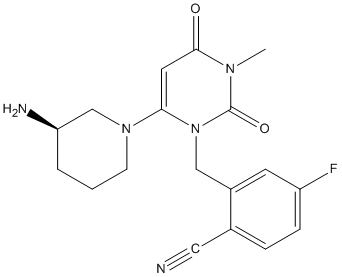
Chemical_Nomenclature : 2-[[6-[(3R)-3-aminopiperidin-1-yl]-3-methyl-2,4-dioxopyrimidin-1-yl]methyl]-4-fluorobenzonitrile
Canonical SMILES : CN1C(=O)C=C(N(C1=O)CC2=C(C=CC(=C2)F)C#N)N3CCCC(C3)N
InChI : InChI=1S\/C18H20FN5O2\/c1-22-17(25)8-16(23-6-2-3-15(21)11-23)24(18(22)26)10-13-7-14(19)5-4-12(13)9-20\/h4-5,7-8,15H,2-3,6,10-11,21H2,1H3\/t15-\/m1\/s1
InChIKey : IWYJYHUNXVAVAA-OAHLLOKOSA-N
Other name(s) : SYR-472,SYR-472, Zafatek,Trelagliptin(SYR-472),UNII-Q836OWG55H,Trelagliptin [USAN]
MW : 357.38
Formula : C18H20FN5O2
CAS_number : 865759-25-7
PubChem : 15983988
UniChem : IWYJYHUNXVAVAA-OAHLLOKOSA-N
IUPHAR :
Wikipedia :
Target
Families : Trelagliptin ligand of proteins in family: DPP4N_Peptidase_S9
Stucture : 5KBY Crystal structure of dipeptidyl peptidase IV in complex with SYR-472
Protein : human-DPP4
References (7)
| Title : Trelagliptin ameliorates IL-1beta-impaired chondrocyte function via the AMPK\/SOX-9 pathway - Liu_2021_Mol.Immunol_140_70 |
| Author(s) : Liu J , Zuo Q , Li Z , Chen J , Liu F |
| Ref : Mol Immunol , 140 :70 , 2021 |
| Abstract : Liu_2021_Mol.Immunol_140_70 |
| ESTHER : Liu_2021_Mol.Immunol_140_70 |
| PubMedSearch : Liu_2021_Mol.Immunol_140_70 |
| PubMedID: 34666245 |
| Title : Safety evaluation of trelagliptin in the treatment of Japanese type 2 diabetes mellitus patients - Kaku_2017_Expert.Opin.Drug.Saf_16_1313 |
| Author(s) : Kaku K |
| Ref : Expert Opin Drug Safety , 16 :1313 , 2017 |
| Abstract : Kaku_2017_Expert.Opin.Drug.Saf_16_1313 |
| ESTHER : Kaku_2017_Expert.Opin.Drug.Saf_16_1313 |
| PubMedSearch : Kaku_2017_Expert.Opin.Drug.Saf_16_1313 |
| PubMedID: 28829213 |
| Title : Effect of trelagliptin on vascular endothelial functions and serum adiponectin level in patients with type 2 diabetes: a preliminary single-arm prospective pilot study - Ida_2016_Cardiovasc.Diabetol_15_153 |
| Author(s) : Ida S , Murata K , Betou K , Kobayashi C , Ishihara Y , Imataka K , Uchida A , Monguchi K , Kaneko R , Fujiwara R , Takahashi H |
| Ref : Cardiovasc Diabetol , 15 :153 , 2016 |
| Abstract : Ida_2016_Cardiovasc.Diabetol_15_153 |
| ESTHER : Ida_2016_Cardiovasc.Diabetol_15_153 |
| PubMedSearch : Ida_2016_Cardiovasc.Diabetol_15_153 |
| PubMedID: 27809903 |
| Title : Trelagliptin (SYR-472, Zafatek), Novel Once-Weekly Treatment for Type 2 Diabetes, Inhibits Dipeptidyl Peptidase-4 (DPP-4) via a Non-Covalent Mechanism - Grimshaw_2016_PLoS.One_11_e0157509 |
| Author(s) : Grimshaw CE , Jennings A , Kamran R , Ueno H , Nishigaki N , Kosaka T , Tani A , Sano H , Kinugawa Y , Koumura E , Shi L , Takeuchi K |
| Ref : PLoS ONE , 11 :e0157509 , 2016 |
| Abstract : Grimshaw_2016_PLoS.One_11_e0157509 |
| ESTHER : Grimshaw_2016_PLoS.One_11_e0157509 |
| PubMedSearch : Grimshaw_2016_PLoS.One_11_e0157509 |
| PubMedID: 27328054 |
| Gene_locus related to this paper: human-DPP4 |
| Title : First novel once-weekly DPP-4 inhibitor, trelagliptin, for the treatment of type 2 diabetes mellitus - Kaku_2015_Expert.Opin.Pharmacother_16_2539 |
| Author(s) : Kaku K |
| Ref : Expert Opin Pharmacother , 16 :2539 , 2015 |
| Abstract : Kaku_2015_Expert.Opin.Pharmacother_16_2539 |
| ESTHER : Kaku_2015_Expert.Opin.Pharmacother_16_2539 |
| PubMedSearch : Kaku_2015_Expert.Opin.Pharmacother_16_2539 |
| PubMedID: 26523434 |
| Title : Trelagliptin: First Global Approval - McKeage_2015_Drugs_75_1161 |
| Author(s) : McKeage K |
| Ref : Drugs , 75 :1161 , 2015 |
| Abstract : McKeage_2015_Drugs_75_1161 |
| ESTHER : McKeage_2015_Drugs_75_1161 |
| PubMedSearch : McKeage_2015_Drugs_75_1161 |
| PubMedID: 26115728 |
| Title : SYR-472, a novel once-weekly dipeptidyl peptidase-4 (DPP-4) inhibitor, in type 2 diabetes mellitus: a phase 2, randomised, double-blind, placebo-controlled trial - Inagaki_2014_Lancet.Diabetes.Endocrinol_2_125 |
| Author(s) : Inagaki N , Onouchi H , Sano H , Funao N , Kuroda S , Kaku K |
| Ref : Lancet Diabetes Endocrinol , 2 :125 , 2014 |
| Abstract : Inagaki_2014_Lancet.Diabetes.Endocrinol_2_125 |
| ESTHER : Inagaki_2014_Lancet.Diabetes.Endocrinol_2_125 |
| PubMedSearch : Inagaki_2014_Lancet.Diabetes.Endocrinol_2_125 |
| PubMedID: 24622716 |