Trifluoperazine
It has a role as a dopaminergic antagonist, an antiemetic, an EC 1.8.1.12 (trypanothione-disulfide reductase) inhibitor, an EC 5.3.3.5 (cholestenol Delta-isomerase) inhibitor, a calmodulin antagonist and a phenothiazine antipsychotic drug no longer commonly used in clinical practice
General
Type : Phenothiazine,Sulfur Compound,Trifluoro,Not A\/B H target
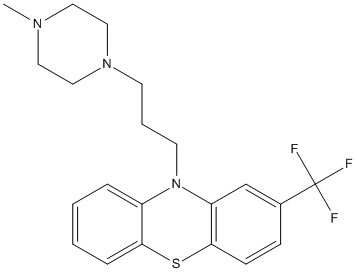
Chemical_Nomenclature : 10-[3-(4-methylpiperazin-1-yl)propyl]-2-(trifluoromethyl)phenothiazine
Canonical SMILES : CN1CCN(CC1)CCCN2C3=CC=CC=C3SC4=C2C=C(C=C4)C(F)(F)F
InChI : InChI=1S\/C21H24F3N3S\/c1-25-11-13-26(14-12-25)9-4-10-27-17-5-2-3-6-19(17)28-20-8-7-16(15-18(20)27)21(22,23)24\/h2-3,5-8,15H,4,9-14H2,1H3
InChIKey : ZEWQUBUPAILYHI-UHFFFAOYSA-N
Other name(s) : Trifluperazine,Trifluoroperazine,Trifluoperazin,CHEBI:45951,SCHEMBL24866,ZINC19418959,DB00831
MW : 407.5
Formula : C21H24F3N3S
CAS_number : 117-89-5
PubChem : 5566
UniChem : ZEWQUBUPAILYHI-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
Families :
Stucture :
Protein :
References (11)
| Title : Involvement of calmodulin inhibition in analgesia induced with low doses of intrathecal trifluoperazine - Golbidi_2002_Jpn.J.Pharmacol_88_151 |
| Author(s) : Golbidi S , Moriuchi H , Irie T , Ghafghazi T , Hajhashemi V |
| Ref : Japanese Journal of Pharmacology , 88 :151 , 2002 |
| Abstract : Golbidi_2002_Jpn.J.Pharmacol_88_151 |
| ESTHER : Golbidi_2002_Jpn.J.Pharmacol_88_151 |
| PubMedSearch : Golbidi_2002_Jpn.J.Pharmacol_88_151 |
| PubMedID: 11928715 |
| Title : Inhibition of butyrylcholinesterase by phenothiazine derivatives - Debord_2002_J.Enzyme.Inhib.Med.Chem_17_197 |
| Author(s) : Debord J , Merle L , Bollinger JC , Dantoine T |
| Ref : J Enzyme Inhib Med Chem , 17 :197 , 2002 |
| Abstract : Debord_2002_J.Enzyme.Inhib.Med.Chem_17_197 |
| ESTHER : Debord_2002_J.Enzyme.Inhib.Med.Chem_17_197 |
| PubMedSearch : Debord_2002_J.Enzyme.Inhib.Med.Chem_17_197 |
| PubMedID: 12443046 |
| Title : Effects of trifluoperazine on synaptically evoked potentials and membrane properties of CA1 pyramidal neurons of rat hippocampus in situ and in vitro - Agopyan_1993_Synapse_13_10 |
| Author(s) : Agopyan N , Krnjevic K |
| Ref : Synapse , 13 :10 , 1993 |
| Abstract : Agopyan_1993_Synapse_13_10 |
| ESTHER : Agopyan_1993_Synapse_13_10 |
| PubMedSearch : Agopyan_1993_Synapse_13_10 |
| PubMedID: 7678946 |
| Title : Poster: Inhibition of human erythrocyte DSAChE by chlorpromazine and trifluoperazine is Independent from drug effects on membrane environment - |
| Author(s) : Spinedi A , Pacini L , Luly P |
| Ref : In: Cholinesterases: Structure, Function, Mechanism, Genetics, and Cell Biology , (Massoulie J, Barnard EA, Chatonnet A, Bacou F, Doctor BP, Quinn DM) American Chemical Society, Washington, DC :344 , 1991 |
| PubMedID: |
| Title : Synergistic inhibition by trifluoperazine and phencyclidine of carbamylcholine-induced cation influx in muscle cultures - Gamliel_1987_Biochem.Pharmacol_36_2271 |
| Author(s) : Gamliel A , Schreiber G , Shainberg A |
| Ref : Biochemical Pharmacology , 36 :2271 , 1987 |
| Abstract : Gamliel_1987_Biochem.Pharmacol_36_2271 |
| ESTHER : Gamliel_1987_Biochem.Pharmacol_36_2271 |
| PubMedSearch : Gamliel_1987_Biochem.Pharmacol_36_2271 |
| PubMedID: 3606641 |
| Title : Trifluoperazine and phencyclidine inhibit synergistically carbamylcholine-induced cation influx in muscle cultures - Shainberg_1987_Prog.Clin.Biol.Res_253_287 |
| Author(s) : Shainberg A , Gamliel A , Schreiber G |
| Ref : Prog Clin Biol Res , 253 :287 , 1987 |
| Abstract : Shainberg_1987_Prog.Clin.Biol.Res_253_287 |
| ESTHER : Shainberg_1987_Prog.Clin.Biol.Res_253_287 |
| PubMedSearch : Shainberg_1987_Prog.Clin.Biol.Res_253_287 |
| PubMedID: 3432292 |
| Title : The effects of anticytoskeletal drugs and of trifluoperazine on the release of preformed and newly synthesized acetylcholine - |
| Author(s) : Michaelson DM , Luz S , Pinchasi I |
| Ref : Rev Clin Basic Pharm , 6 Suppl :1S , 1986 |
| PubMedID: 3615962 |
| Title : Inhibition of myogenesis by trifluoperazine and compound 48\/80 - Shainberg_1985_Cell.Biol.Int.Rep_9_265 |
| Author(s) : Shainberg A , Cahan R |
| Ref : Cell Biology International Report , 9 :265 , 1985 |
| Abstract : Shainberg_1985_Cell.Biol.Int.Rep_9_265 |
| ESTHER : Shainberg_1985_Cell.Biol.Int.Rep_9_265 |
| PubMedSearch : Shainberg_1985_Cell.Biol.Int.Rep_9_265 |
| PubMedID: 3886169 |
| Title : Trifluoperazine reduces inward ionic currents and secretion by separate mechanisms in bovine chromaffin cells - Clapham_1984_J.Physiol_353_541 |
| Author(s) : Clapham DE , Neher E |
| Ref : The Journal of Physiology , 353 :541 , 1984 |
| Abstract : Clapham_1984_J.Physiol_353_541 |
| ESTHER : Clapham_1984_J.Physiol_353_541 |
| PubMedSearch : Clapham_1984_J.Physiol_353_541 |
| PubMedID: 6090644 |
| Title : Trifluoperazine stimulates acetylcholine receptor synthesis in cultured chick myotubes - Schneider_1984_J.Neurochem_42_1395 |
| Author(s) : Schneider M , Shieh BH , Pezzementi L , Schmidt J |
| Ref : Journal of Neurochemistry , 42 :1395 , 1984 |
| Abstract : Schneider_1984_J.Neurochem_42_1395 |
| ESTHER : Schneider_1984_J.Neurochem_42_1395 |
| PubMedSearch : Schneider_1984_J.Neurochem_42_1395 |
| PubMedID: 6142923 |
| Title : Trifluoperazine, a calmodulin antagonist, inhibits muscle cell fusion - Bar-Sagi_1983_J.Cell.Biol_97_1375 |
| Author(s) : Bar-Sagi D , Prives J |
| Ref : Journal of Cell Biology , 97 :1375 , 1983 |
| Abstract : Bar-Sagi_1983_J.Cell.Biol_97_1375 |
| ESTHER : Bar-Sagi_1983_J.Cell.Biol_97_1375 |
| PubMedSearch : Bar-Sagi_1983_J.Cell.Biol_97_1375 |
| PubMedID: 6415064 |