Triflupromazine
Dopaminergic antagonist, an antiemetic, a first generation antipsychotic and an anticoronaviral agent
General
Type : Phenothiazine,Sulfur Compound,Trifluoro,Not A\/B H target
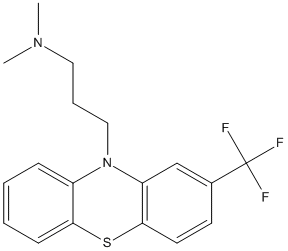
Chemical_Nomenclature : N,N-dimethyl-3-[2-(trifluoromethyl)phenothiazin-10-yl]propan-1-amine
Canonical SMILES : CN(C)CCCN1C2=CC=CC=C2SC3=C1C=C(C=C3)C(F)(F)F
InChI : InChI=1S\/C18H19F3N2S\/c1-22(2)10-5-11-23-14-6-3-4-7-16(14)24-17-9-8-13(12-15(17)23)18(19,20)21\/h3-4,6-9,12H,5,10-11H2,1-2H3
InChIKey : XSCGXQMFQXDFCW-UHFFFAOYSA-N
Other name(s) : triflupromazine,Fluopromazine,Trifluopromazine,Vesprin,Adazine,CHEBI:9711,CHEMBL570,SCHEMBL44085,ZINC538507
MW : 352.4
Formula : C18H19F3N2S
CAS_number : 146-54-3
PubChem : 5568
UniChem : XSCGXQMFQXDFCW-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
Families :
Stucture :
Protein :
References (4)
| Title : Inactivation of cholinesterase induced by chlorpromazine cation radicals - Muraoka_2003_Pharmacol.Toxicol_92_100 |
| Author(s) : Muraoka S , Miura T |
| Ref : Pharmacol Toxicol , 92 :100 , 2003 |
| Abstract : Muraoka_2003_Pharmacol.Toxicol_92_100 |
| ESTHER : Muraoka_2003_Pharmacol.Toxicol_92_100 |
| PubMedSearch : Muraoka_2003_Pharmacol.Toxicol_92_100 |
| PubMedID: 12747580 |
| Title : Inhibition of butyrylcholinesterase by phenothiazine derivatives - Debord_2002_J.Enzyme.Inhib.Med.Chem_17_197 |
| Author(s) : Debord J , Merle L , Bollinger JC , Dantoine T |
| Ref : J Enzyme Inhib Med Chem , 17 :197 , 2002 |
| Abstract : Debord_2002_J.Enzyme.Inhib.Med.Chem_17_197 |
| ESTHER : Debord_2002_J.Enzyme.Inhib.Med.Chem_17_197 |
| PubMedSearch : Debord_2002_J.Enzyme.Inhib.Med.Chem_17_197 |
| PubMedID: 12443046 |
| Title : Influence of dietary protein on the effect of coumaphos and triflupromazine interaction in sheep - Gopal_1976_Am.J.Vet.Res_37_1143 |
| Author(s) : Gopal T , Oehme FW , Omer S |
| Ref : American Journal of Veterinary Research , 37 :1143 , 1976 |
| Abstract : Gopal_1976_Am.J.Vet.Res_37_1143 |
| ESTHER : Gopal_1976_Am.J.Vet.Res_37_1143 |
| PubMedSearch : Gopal_1976_Am.J.Vet.Res_37_1143 |
| PubMedID: 984539 |
| Title : Central and peripheral actions of anticholinergic drugs when administered with triflupromazine - |
| Author(s) : Brimblecombe RW , Green DM , Aldous FA , Thompson PB |
| Ref : Neuropharmacology , 10 :93 , 1971 |
| PubMedID: 5569310 |