WWL70
(Differs from WWL123 only by a pyridine instead of a phenyl) Potent inhibitor of ABHD6 (IC50=70 nM) hydrolysis of 2-arachidonylglycerol. Inhibits also CES1 Iglesias et al.
General
Type : Carbamate,Biphenyl
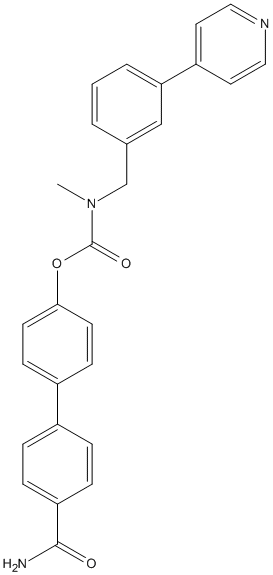
Chemical_Nomenclature : [4-(4-carbamoylphenyl)phenyl] N-methyl-N-[(3-pyridin-4-ylphenyl)methyl]carbamate
Canonical SMILES : CN(CC1=CC=CC(=C1)C2=CC=NC=C2)C(=O)OC3=CC=C(C=C3)C4=CC=C(C=C4)C(=O)N
InChI : InChI=1S\/C27H23N3O3\/c1-30(18-19-3-2-4-24(17-19)22-13-15-29-16-14-22)27(32)33-25-11-9-21(10-12-25)20-5-7-23(8-6-20)26(28)31\/h2-17H,18H2,1H3,(H2,28,31)
InChIKey : QTWNORFUQILKJL-UHFFFAOYSA-N
Other name(s) : WWL-70,WWL 70,947669-91-2,GTPL5289,SCHEMBL14684619,CTK8E9903,N-Methyl-N-[[3-(4-pyridinyl)phenyl]methyl]-4'-(aminocarbonyl)[1,1'-biphenyl]-4-yl carbamic acid ester
MW : 437.49
Formula : C27H23N3O3
CAS_number : 947669-91-2
PubChem : 17759121
UniChem : QTWNORFUQILKJL-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
Families : WWL70 ligand of proteins in family: ABHD6-Lip || Carb_B_Chordata
Stucture :
Protein : human-ABHD6
References (10)
| Title : Wwl70-induced ABHD6 inhibition attenuates memory deficits and pathological phenotypes in APPswe\/PS1dE9 mice - Xue_2023_Pharmacol.Res__106864 |
| Author(s) : Xue Z , Ye L , Ge J , Lan Z , Zou X , Mao C , Bao X , Yu L , Xu Y , Zhu X |
| Ref : Pharmacol Res , :106864 , 2023 |
| Abstract : Xue_2023_Pharmacol.Res__106864 |
| ESTHER : Xue_2023_Pharmacol.Res__106864 |
| PubMedSearch : Xue_2023_Pharmacol.Res__106864 |
| PubMedID: 37480972 |
| Gene_locus related to this paper: human-ABHD6 , mouse-ABHD6 |
| Title : The alpha\/beta-hydrolase domain 6 inhibitor WWL70 decreases endotoxin-induced lung inflammation in mice, potential contribution of 2-arachidonoylglycerol, and lysoglycerophospholipids - Bottemanne_2019_FASEB.J__fj201802259R |
| Author(s) : Bottemanne P , Paquot A , Ameraoui H , Alhouayek M , Muccioli GG |
| Ref : FASEB Journal , :fj201802259R , 2019 |
| Abstract : Bottemanne_2019_FASEB.J__fj201802259R |
| ESTHER : Bottemanne_2019_FASEB.J__fj201802259R |
| PubMedSearch : Bottemanne_2019_FASEB.J__fj201802259R |
| PubMedID: 30896979 |
| Gene_locus related to this paper: human-ABHD6 |
| Title : Human leukocytes differentially express endocannabinoid-glycerol lipases and hydrolyze 2-arachidonoyl-glycerol and its metabolites from the 15-lipoxygenase and cyclooxygenase pathways - Turcotte_2019_J.Leukoc.Biol_106_1337 |
| Author(s) : Turcotte C , Dumais E , Archambault AS , Martin C , Blanchet MR , Bissonnette E , Boulet LP , Laviolette M , Di Marzo V , Flamand N |
| Ref : J Leukoc Biol , 106 :1337 , 2019 |
| Abstract : Turcotte_2019_J.Leukoc.Biol_106_1337 |
| ESTHER : Turcotte_2019_J.Leukoc.Biol_106_1337 |
| PubMedSearch : Turcotte_2019_J.Leukoc.Biol_106_1337 |
| PubMedID: 31556464 |
| Gene_locus related to this paper: human-ABHD6 , human-ABHD12 , human-ABHD16A , human-CES1 , human-LYPLA2 , human-PPT1 |
| Title : WWL70 protects against chronic constriction injury-induced neuropathic pain in mice by cannabinoid receptor-independent mechanisms - Wen_2018_J.Neuroinflammation_15_9 |
| Author(s) : Wen J , Jones M , Tanaka M , Selvaraj P , Symes AJ , Cox B , Zhang Y |
| Ref : J Neuroinflammation , 15 :9 , 2018 |
| Abstract : Wen_2018_J.Neuroinflammation_15_9 |
| ESTHER : Wen_2018_J.Neuroinflammation_15_9 |
| PubMedSearch : Wen_2018_J.Neuroinflammation_15_9 |
| PubMedID: 29310667 |
| Title : Anticonvulsive effects of endocannabinoids\; an investigation to determine the role of regulatory components of endocannabinoid metabolism in the Pentylenetetrazol induced tonic- clonic seizures - Zareie_2018_Metab.Brain.Dis_33_939 |
| Author(s) : Zareie P , Sadegh M , Palizvan MR , Moradi-Chameh H |
| Ref : Metabolic Brain Disease , 33 :939 , 2018 |
| Abstract : Zareie_2018_Metab.Brain.Dis_33_939 |
| ESTHER : Zareie_2018_Metab.Brain.Dis_33_939 |
| PubMedSearch : Zareie_2018_Metab.Brain.Dis_33_939 |
| PubMedID: 29504066 |
| Title : WWL70 attenuates PGE2 production derived from 2-arachidonoylglycerol in microglia by ABHD6-independent mechanism - Tanaka_2017_J.Neuroinflammation_14_7 |
| Author(s) : Tanaka M , Moran S , Wen J , Affram K , Chen T , Symes AJ , Zhang Y |
| Ref : J Neuroinflammation , 14 :7 , 2017 |
| Abstract : Tanaka_2017_J.Neuroinflammation_14_7 |
| ESTHER : Tanaka_2017_J.Neuroinflammation_14_7 |
| PubMedSearch : Tanaka_2017_J.Neuroinflammation_14_7 |
| PubMedID: 28086912 |
| Title : Simplified assays of lipolysis enzymes for drug discovery and specificity assessment of known inhibitors - Iglesias_2016_J.Lipid.Res_57_131 |
| Author(s) : Iglesias J , Lamontagne J , Erb H , Gezzar S , Zhao S , Joly E , Truong VL , Skorey K , Crane S , Madiraju SR , Prentki M |
| Ref : J Lipid Res , 57 :131 , 2016 |
| Abstract : Iglesias_2016_J.Lipid.Res_57_131 |
| ESTHER : Iglesias_2016_J.Lipid.Res_57_131 |
| PubMedSearch : Iglesias_2016_J.Lipid.Res_57_131 |
| PubMedID: 26423520 |
| Title : Activation of CB2 receptor is required for the therapeutic effect of ABHD6 inhibition in experimental autoimmune encephalomyelitis - Wen_2015_Neuropharmacol_99_196 |
| Author(s) : Wen J , Ribeiro R , Tanaka M , Zhang Y |
| Ref : Neuropharmacology , 99 :196 , 2015 |
| Abstract : Wen_2015_Neuropharmacol_99_196 |
| ESTHER : Wen_2015_Neuropharmacol_99_196 |
| PubMedSearch : Wen_2015_Neuropharmacol_99_196 |
| PubMedID: 26189763 |
| Title : Selective inhibition of alpha\/beta-hydrolase domain 6 attenuates neurodegeneration, alleviates blood brain barrier breakdown, and improves functional recovery in a mouse model of traumatic brain injury - Tchantchou_2013_J.Neurotrauma_30_565 |
| Author(s) : Tchantchou F , Zhang Y |
| Ref : Journal of Neurotrauma , 30 :565 , 2013 |
| Abstract : Tchantchou_2013_J.Neurotrauma_30_565 |
| ESTHER : Tchantchou_2013_J.Neurotrauma_30_565 |
| PubMedSearch : Tchantchou_2013_J.Neurotrauma_30_565 |
| PubMedID: 23151067 |
| Title : Monoacylglycerol lipase limits the duration of endocannabinoid-mediated depolarization-induced suppression of excitation in autaptic hippocampal neurons - Straiker_2009_Mol.Pharmacol_76_1220 |
| Author(s) : Straiker A , Hu SS , Long JZ , Arnold A , Wager-Miller J , Cravatt BF , Mackie K |
| Ref : Molecular Pharmacology , 76 :1220 , 2009 |
| Abstract : Straiker_2009_Mol.Pharmacol_76_1220 |
| ESTHER : Straiker_2009_Mol.Pharmacol_76_1220 |
| PubMedSearch : Straiker_2009_Mol.Pharmacol_76_1220 |
| PubMedID: 19767452 |
| Gene_locus related to this paper: human-MGLL |