Wedelolactone
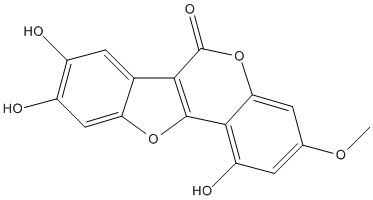
Wedelolactone is a natural product, coumestan with hydroxy substituents as positions 1, 8 and 9 and a methoxy substituent at position 3. It has a role as an antineoplastic agent, an EC 5.99.1.3 [DNA topoisomerase (ATP-hydrolysing)] inhibitor, an apoptosis inducer, a hepatoprotective agent and a metabolite
General
Type : Natural
Chemical_Nomenclature : 1,8,9-trihydroxy-3-methoxy-[1]benzofuro[3,2-c]chromen-6-one
Canonical SMILES : COC1=CC(=C2C(=C1)OC(=O)C3=C2OC4=CC(=C(C=C43)O)O)O
InChI : InChI=1S\/C16H10O7\/c1-21-6-2-10(19)14-12(3-6)23-16(20)13-7-4-8(17)9(18)5-11(7)22-15(13)14\/h2-5,17-19H,1H3
InChIKey : XQDCKJKKMFWXGB-UHFFFAOYSA-N
Other name(s) : 7-Methoxy-5,11,12-trihydroxycoumestan,7-Methoxy-5,11,12-trihydroxy-coumestan,IKK Inhibitor II,CHEMBL97453,SCHEMBL601220,ZINC6483512
MW : 314.25
Formula : C16H10O7
CAS_number : 524-12-9
PubChem : 5281813
UniChem : XQDCKJKKMFWXGB-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
Families : Wedelolactone ligand of proteins in family: Epoxide_hydrolase
Stucture :
Protein : human-EPHX2
References (1)
| Title : Macrophage Inactivation by Small Molecule Wedelolactone via Targeting sEH for the Treatment of LPS-Induced Acute Lung Injury - Zhang_2023_ACS.Cent.Sci_9_440 |
| Author(s) : Zhang J , Zhang M , Huo XK , Ning J , Yu ZL , Morisseau C , Sun CP , Hammock BD , Ma XC |
| Ref : ACS Cent Sci , 9 :440 , 2023 |
| Abstract : Zhang_2023_ACS.Cent.Sci_9_440 |
| ESTHER : Zhang_2023_ACS.Cent.Sci_9_440 |
| PubMedSearch : Zhang_2023_ACS.Cent.Sci_9_440 |
| PubMedID: 36968547 |