Bambuterol
General
Type : Pro-Drug || Drug || Carbamate || tert-Butyl || Adrenergic-Receptor-ligand
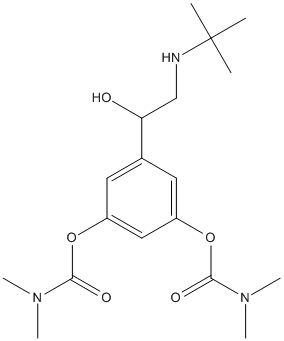
Chemical_Nomenclature : [3-[2-(tert-butylamino)-1-hydroxyethyl]-5-(dimethylcarbamoyloxy)phenyl] N,N-dimethylcarbamate
Canonical SMILES : CC(C)(C)NCC(C1=CC(=CC(=C1)OC(=O)N(C)C)OC(=O)N(C)C)O
InChI : InChI=1S\/C18H29N3O5\/c1-18(2,3)19-11-15(22)12-8-13(25-16(23)20(4)5)10-14(9-12)26-17(24)21(6)7\/h8-10,15,19,22H,11H2,1-7H3
InChIKey : ANZXOIAKUNOVQU-UHFFFAOYSA-N
Other name(s) : bis-dimethyl-carbamate of terbutaline, Bambec, Bambuterolum, Oxeol, Prestwick0_000361, Prestwick1_000361, SPBio_002402
Target
Families : BCHE
References (22)
| Title : Postnatal developmental delay and supersensitivity to organophosphate in gene-targeted mice lacking acetylcholinesterase - Xie_2000_J.Pharmacol.Exp.Ther_293_896 |
| Author(s) : Xie W , Stribley JA , Chatonnet A , Wilder PJ , Rizzino A , McComb RD , Taylor P , Hinrichs SH , Lockridge O |
| Ref : Journal of Pharmacology & Experimental Therapeutics , 293 :896 , 2000 |
| Abstract : Xie_2000_J.Pharmacol.Exp.Ther_293_896 |
| ESTHER : Xie_2000_J.Pharmacol.Exp.Ther_293_896 |
| PubMedSearch : Xie_2000_J.Pharmacol.Exp.Ther_293_896 |
| PubMedID: 10869390 |
| Gene_locus related to this paper: mouse-ACHE |
| Title : The influence of drug-induced low plasma cholinesterase activity on the pharmacokinetics and pharmacodynamics of mivacurium - Ostergaard_2000_Anesthesiology_92_1581 |
| Author(s) : Ostergaard D , Rasmussen SN , Viby-Mogensen J , Pedersen NA , Boysen R |
| Ref : Anesthesiology , 92 :1581 , 2000 |
| Abstract : Ostergaard_2000_Anesthesiology_92_1581 |
| ESTHER : Ostergaard_2000_Anesthesiology_92_1581 |
| PubMedSearch : Ostergaard_2000_Anesthesiology_92_1581 |
| PubMedID: 10839906 |
| Title : Amino acid residues involved in the interaction of acetylcholinesterase and butyrylcholinesterase with the carbamates Ro 02-0683 and bambuterol, and with terbutaline - Kovarik_1999_Biochim.Biophys.Acta_1433_261 |
| Author(s) : Kovarik Z , Radic Z , Grgas B , Skrinjaric-Spoljar M , Reiner E , Simeon-Rudolf V |
| Ref : Biochimica & Biophysica Acta , 1433 :261 , 1999 |
| Abstract : Kovarik_1999_Biochim.Biophys.Acta_1433_261 |
| ESTHER : Kovarik_1999_Biochim.Biophys.Acta_1433_261 |
| PubMedSearch : Kovarik_1999_Biochim.Biophys.Acta_1433_261 |
| PubMedID: 10446376 |
| Title : Pharmacokinetics of bambuterol in subjects homozygous for the atypical gene for plasma cholinesterase - Bang_1998_Br.J.Clin.Pharmacol_45_479 |
| Author(s) : Bang U , Nyberg L , Rosenborg J , Viby-Mogensen J |
| Ref : British Journal of Clinical Pharmacology , 45 :479 , 1998 |
| Abstract : Bang_1998_Br.J.Clin.Pharmacol_45_479 |
| ESTHER : Bang_1998_Br.J.Clin.Pharmacol_45_479 |
| PubMedSearch : Bang_1998_Br.J.Clin.Pharmacol_45_479 |
| PubMedID: 9643621 |
| Title : Pharmacokinetics of bambuterol in healthy subjects - Nyberg_1998_Br.J.Clin.Pharmacol_45_471 |
| Author(s) : Nyberg L , Rosenborg J , Weibull E , Jonsson S , Kennedy BM , Nilsson M |
| Ref : British Journal of Clinical Pharmacology , 45 :471 , 1998 |
| Abstract : Nyberg_1998_Br.J.Clin.Pharmacol_45_471 |
| ESTHER : Nyberg_1998_Br.J.Clin.Pharmacol_45_471 |
| PubMedSearch : Nyberg_1998_Br.J.Clin.Pharmacol_45_471 |
| PubMedID: 9643620 |
| Title : Drug interactions with neuromuscular blockers - Feldman_1996_Drug.Saf_15_261 |
| Author(s) : Feldman S , Karalliedde L |
| Ref : Drug Safety , 15 :261 , 1996 |
| Abstract : Feldman_1996_Drug.Saf_15_261 |
| ESTHER : Feldman_1996_Drug.Saf_15_261 |
| PubMedSearch : Feldman_1996_Drug.Saf_15_261 |
| PubMedID: 8905251 |
| Title : Micro-CLC as an interface between SLM extraction and CZE for enhancement of sensitivity and selectivity in bioanalysis of drugs - Palmarsdottir_1996_J.Capillary.Electrophor_3_255 |
| Author(s) : Palmarsdottir S , Mathiasson L , Jonsson JA , Edholm LE |
| Ref : J Capillary Electrophor , 3 :255 , 1996 |
| Abstract : Palmarsdottir_1996_J.Capillary.Electrophor_3_255 |
| ESTHER : Palmarsdottir_1996_J.Capillary.Electrophor_3_255 |
| PubMedSearch : Palmarsdottir_1996_J.Capillary.Electrophor_3_255 |
| PubMedID: 9384731 |
| Title : Clinical pharmacokinetics of bambuterol - Sitar_1996_Clin.Pharmacokinet_31_246 |
| Author(s) : Sitar DS |
| Ref : Clinical Pharmacokinetics , 31 :246 , 1996 |
| Abstract : Sitar_1996_Clin.Pharmacokinet_31_246 |
| ESTHER : Sitar_1996_Clin.Pharmacokinet_31_246 |
| PubMedSearch : Sitar_1996_Clin.Pharmacokinet_31_246 |
| PubMedID: 8896942 |
| Title : Bambuterol in the treatment of asthma. A placebo-controlled comparison of once-daily morning vs evening administration [see comments] - D'Alonzo_1995_Chest_107_406 |
| Author(s) : D'Alonzo GE , Smolensky MH , Feldman S , Gnosspelius Y , Karlsson K |
| Ref : Chest , 107 :406 , 1995 |
| Abstract : D'Alonzo_1995_Chest_107_406 |
| ESTHER : D'Alonzo_1995_Chest_107_406 |
| PubMedSearch : D'Alonzo_1995_Chest_107_406 |
| PubMedID: 7842769 |
| Title : Terbutaline prodrugs and oral beta 2-agonist therapy - |
| Author(s) : Svensson LA |
| Ref : Pharmacol Toxicol , 77 Suppl 3 :30 , 1995 |
| PubMedID: 8751147 |
| Title : Clinical importance of plasma cholinesterase for the anaesthetist. - Pedersen_1994_Ann.Acad.Med.Singapore_23_120 |
| Author(s) : Pedersen NA , Jensen FS |
| Ref : Annals of the Academy of Medicine, Singapore , 23 :120 , 1994 |
| Abstract : Pedersen_1994_Ann.Acad.Med.Singapore_23_120 |
| ESTHER : Pedersen_1994_Ann.Acad.Med.Singapore_23_120 |
| PubMedSearch : Pedersen_1994_Ann.Acad.Med.Singapore_23_120 |
| PubMedID: 7710221 |
| Title : Cholinesterases regulate neurite growth of chick nerve cells in vitro by means of a non-enzymatic mechanism - Layer_1993_Cell.Tiss.Res_273_219 |
| Author(s) : Layer PG , Weikert T , Alber R |
| Ref : Cell & Tissue Research , 273 :219 , 1993 |
| Abstract : Layer_1993_Cell.Tiss.Res_273_219 |
| ESTHER : Layer_1993_Cell.Tiss.Res_273_219 |
| PubMedSearch : Layer_1993_Cell.Tiss.Res_273_219 |
| PubMedID: 8103422 |
| Title : Chimeric human cholinesterase. Identification of interaction sites responsible for recognition of acetyl- or butyrylcholinesterase-specific ligands - Loewenstein_1993_J.Mol.Biol_234_289 |
| Author(s) : Loewenstein Y , Gnatt A , Neville LF , Soreq H |
| Ref : Journal of Molecular Biology , 234 :289 , 1993 |
| Abstract : Loewenstein_1993_J.Mol.Biol_234_289 |
| ESTHER : Loewenstein_1993_J.Mol.Biol_234_289 |
| PubMedSearch : Loewenstein_1993_J.Mol.Biol_234_289 |
| PubMedID: 8230213 |
| Title : Structure-function relationship studies in human cholinesterases reveal genomic origins for individual variations in cholinergic drug responses. - Loewenstein_1993_Prog.Neuropsychopharmacol.Biol.Psychiatry_17_905 |
| Author(s) : Loewenstein Y , Gnatt A , Neville LF , Zakut H , Soreq H |
| Ref : Prog Neuropsychopharmacol Biological Psychiatry , 17 :905 , 1993 |
| Abstract : Loewenstein_1993_Prog.Neuropsychopharmacol.Biol.Psychiatry_17_905 |
| ESTHER : Loewenstein_1993_Prog.Neuropsychopharmacol.Biol.Psychiatry_17_905 |
| PubMedSearch : Loewenstein_1993_Prog.Neuropsychopharmacol.Biol.Psychiatry_17_905 |
| PubMedID: 8278601 |
| Title : A placebo-controlled dose-finding study with bambuterol in elderly patients with asthma - Sitar_1993_Chest_103_771 |
| Author(s) : Sitar DS , Aoki FY , Warren CP , Knight A , Grossman RF , Alexander M , Soliman S |
| Ref : Chest , 103 :771 , 1993 |
| Abstract : Sitar_1993_Chest_103_771 |
| ESTHER : Sitar_1993_Chest_103_771 |
| PubMedSearch : Sitar_1993_Chest_103_771 |
| PubMedID: 8449067 |
| Title : Genetic variants of human serum cholinesterase influence metabolism of the muscle relaxant succinylcholine. - Lockridge_1990_Pharmacol.Ther_47_35 |
| Author(s) : Lockridge O |
| Ref : Pharmacol Ther , 47 :35 , 1990 |
| Abstract : Lockridge_1990_Pharmacol.Ther_47_35 |
| ESTHER : Lockridge_1990_Pharmacol.Ther_47_35 |
| PubMedSearch : Lockridge_1990_Pharmacol.Ther_47_35 |
| PubMedID: 2195556 |
| Gene_locus related to this paper: human-BCHE |
| Title : The effect of bambuterol (carbamylated terbutaline) on plasma cholinesterase activity and suxamethonium-induced neuromuscular blockade in genotypically normal patients - Bang_1990_Acta.Anaesthesiol.Scand_34_596 |
| Author(s) : Bang U , Viby-Mogensen J , Wiren JE , Skovgaard LT |
| Ref : Acta Anaesthesiologica Scandinavica , 34 :596 , 1990 |
| Abstract : Bang_1990_Acta.Anaesthesiol.Scand_34_596 |
| ESTHER : Bang_1990_Acta.Anaesthesiol.Scand_34_596 |
| PubMedSearch : Bang_1990_Acta.Anaesthesiol.Scand_34_596 |
| PubMedID: 2244449 |
| Title : The effect of bambuterol on plasma cholinesterase activity and suxamethonium-induced neuromuscular blockade in subjects heterozygous for abnormal plasma cholinesterase - Bang_1990_Acta.Anaesthesiol.Scand_34_600 |
| Author(s) : Bang U , Viby-Mogensen J , Wiren JE |
| Ref : Acta Anaesthesiologica Scandinavica , 34 :600 , 1990 |
| Abstract : Bang_1990_Acta.Anaesthesiol.Scand_34_600 |
| ESTHER : Bang_1990_Acta.Anaesthesiol.Scand_34_600 |
| PubMedSearch : Bang_1990_Acta.Anaesthesiol.Scand_34_600 |
| PubMedID: 2244450 |
| Title : Bambuterol, a carbamate ester prodrug of terbutaline, as inhibitor of cholinesterases in human blood - Tunek_1988_Drug.Metab.Dispos_16_759 |
| Author(s) : Tunek A , Svensson LA |
| Ref : Drug Metabolism & Disposition: The Biological Fate of Chemicals , 16 :759 , 1988 |
| Abstract : Tunek_1988_Drug.Metab.Dispos_16_759 |
| ESTHER : Tunek_1988_Drug.Metab.Dispos_16_759 |
| PubMedSearch : Tunek_1988_Drug.Metab.Dispos_16_759 |
| PubMedID: 2906603 |
| Title : Interaction between bambuterol and physostigmine: aspects on cholinesterase inhibition and neuromuscular transmission in the smooth and skeletal muscles of the guinea-pig - Jeppsson_1988_Pharmacol.Toxicol_63_211 |
| Author(s) : Jeppsson AB , Waldeck B |
| Ref : Pharmacol Toxicol , 63 :211 , 1988 |
| Abstract : Jeppsson_1988_Pharmacol.Toxicol_63_211 |
| ESTHER : Jeppsson_1988_Pharmacol.Toxicol_63_211 |
| PubMedSearch : Jeppsson_1988_Pharmacol.Toxicol_63_211 |
| PubMedID: 2848230 |
| Title : Hydrolysis of 3H-bambuterol, a carbamate prodrug of terbutaline, in blood from humans and laboratory animals in vitro - Tunek_1988_Biochem.Pharmacol_37_3867 |
| Author(s) : Tunek A , Levin E , Svensson LA |
| Ref : Biochemical Pharmacology , 37 :3867 , 1988 |
| Abstract : Tunek_1988_Biochem.Pharmacol_37_3867 |
| ESTHER : Tunek_1988_Biochem.Pharmacol_37_3867 |
| PubMedSearch : Tunek_1988_Biochem.Pharmacol_37_3867 |
| PubMedID: 3190733 |
| Title : New lipophilic terbutaline ester prodrugs with long effect duration - Olsson_1984_Pharm.Res_1_19 |
| Author(s) : Olsson OA , Svensson LA |
| Ref : Pharm Res , 1 :19 , 1984 |
| Abstract : Olsson_1984_Pharm.Res_1_19 |
| ESTHER : Olsson_1984_Pharm.Res_1_19 |
| PubMedSearch : Olsson_1984_Pharm.Res_1_19 |
| PubMedID: 24277179 |