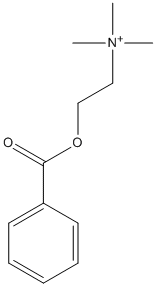
Benzoylcholine
General
Type : Trimethylammonium || Benzoate || Choline ester
Chemical_Nomenclature : 2-benzoyloxyethyl(trimethyl)azanium
Canonical SMILES : C[N+](C)(C)CCOC(=O)C1=CC=CC=C1
InChI : InChI=1S\/C12H18NO2\/c1-13(2,3)9-10-15-12(14)11-7-5-4-6-8-11\/h4-8H,9-10H2,1-3H3\/q+1
InChIKey : HOPVGFKDVOOCHD-UHFFFAOYSA-N
Other name(s) : Choline benzoate, Ethanaminium, TimTec1_001325, 2-(benzoyloxy)-N,N,N-trimethyl-
References (11)
| Title : Hydrolysis of oxo- and thio-esters by human butyrylcholinesterase - Masson_2007_Biochim.Biophys.Acta_1774_16 |
| Author(s) : Masson P , Froment MT , Gillon E , Nachon F , Lockridge O , Schopfer LM |
| Ref : Biochimica & Biophysica Acta , 1774 :16 , 2007 |
| Abstract : Masson_2007_Biochim.Biophys.Acta_1774_16 |
| ESTHER : Masson_2007_Biochim.Biophys.Acta_1774_16 |
| PubMedSearch : Masson_2007_Biochim.Biophys.Acta_1774_16 |
| PubMedID: 17182295 |
| Title : Development and application of serum cholinesterase activity measurement using benzoylthiocholine iodide - Osawa_2005_Clin.Chim.Acta_351_65 |
| Author(s) : Osawa S , Kariyone K , Ichihara F , Arai K , Takagasa N , Ito H |
| Ref : Clinica Chimica Acta , 351 :65 , 2005 |
| Abstract : Osawa_2005_Clin.Chim.Acta_351_65 |
| ESTHER : Osawa_2005_Clin.Chim.Acta_351_65 |
| PubMedSearch : Osawa_2005_Clin.Chim.Acta_351_65 |
| PubMedID: 15563872 |
| Title : Rate-determining step of butyrylcholinesterase-catalyzed hydrolysis of benzoylcholine and benzoylthiocholine. Volumetric study of wild-type and D70G mutant behavior - Masson_2004_Eur.J.Biochem_271_1980 |
| Author(s) : Masson P , Bec N , Froment MT , Nachon F , Balny C , Lockridge O , Schopfer LM |
| Ref : European Journal of Biochemistry , 271 :1980 , 2004 |
| Abstract : Masson_2004_Eur.J.Biochem_271_1980 |
| ESTHER : Masson_2004_Eur.J.Biochem_271_1980 |
| PubMedSearch : Masson_2004_Eur.J.Biochem_271_1980 |
| PubMedID: 15128307 |
| Title : Damped oscillatory hysteretic behaviour of butyrylcholinesterase with benzoylcholine as substrate - Masson_2004_Eur.J.Biochem_271_220 |
| Author(s) : Masson P , Goldstein BN , Debouzy JC , Froment MT , Lockridge O , Schopfer LM |
| Ref : European Journal of Biochemistry , 271 :220 , 2004 |
| Abstract : Masson_2004_Eur.J.Biochem_271_220 |
| ESTHER : Masson_2004_Eur.J.Biochem_271_220 |
| PubMedSearch : Masson_2004_Eur.J.Biochem_271_220 |
| PubMedID: 14686935 |
| Title : Identification of human plasma cholinesterase variants in 6,688 individuals using biochemical analysis - Jensen_1995_Acta.Anaesthesiol.Scand_39_157 |
| Author(s) : Jensen FS , Skovgaard LT , Viby-Mogensen J |
| Ref : Acta Anaesthesiologica Scandinavica , 39 :157 , 1995 |
| Abstract : Jensen_1995_Acta.Anaesthesiol.Scand_39_157 |
| ESTHER : Jensen_1995_Acta.Anaesthesiol.Scand_39_157 |
| PubMedSearch : Jensen_1995_Acta.Anaesthesiol.Scand_39_157 |
| PubMedID: 7793180 |
| Title : Genetic variants of human serum cholinesterase influence metabolism of the muscle relaxant succinylcholine. - Lockridge_1990_Pharmacol.Ther_47_35 |
| Author(s) : Lockridge O |
| Ref : Pharmacol Ther , 47 :35 , 1990 |
| Abstract : Lockridge_1990_Pharmacol.Ther_47_35 |
| ESTHER : Lockridge_1990_Pharmacol.Ther_47_35 |
| PubMedSearch : Lockridge_1990_Pharmacol.Ther_47_35 |
| PubMedID: 2195556 |
| Gene_locus related to this paper: human-BCHE |
| Title : Application of stepwise discriminant analysis in the phenotyping of plasma cholinesterase variants - Turner_1985_Ann.Clin.Biochem_22_175 |
| Author(s) : Turner JM , Hall RA , Whittaker M , Holder RL , Kricka LJ |
| Ref : Annals of Clinical Biochemistry , 22 :175 , 1985 |
| Abstract : Turner_1985_Ann.Clin.Biochem_22_175 |
| ESTHER : Turner_1985_Ann.Clin.Biochem_22_175 |
| PubMedSearch : Turner_1985_Ann.Clin.Biochem_22_175 |
| PubMedID: 4004108 |
| Title : Comparison of a commercially available assay system with two reference methods for the determination of plasma cholinesterase variants - Whittaker_1983_Clin.Chem_29_1746 |
| Author(s) : Whittaker M , Britten JJ , Dawson PJ |
| Ref : Clinical Chemistry , 29 :1746 , 1983 |
| Abstract : Whittaker_1983_Clin.Chem_29_1746 |
| ESTHER : Whittaker_1983_Clin.Chem_29_1746 |
| PubMedSearch : Whittaker_1983_Clin.Chem_29_1746 |
| PubMedID: 6616819 |
| Title : A comparison of some methods of phenotyping the plasma cholinesterase variants using benzoylcholine as substrate - Whittaker_1981_Ann.Clin.Biochem_18_9 |
| Author(s) : Whittaker M , Britten JJ |
| Ref : Annals of Clinical Biochemistry , 18 :9 , 1981 |
| Abstract : Whittaker_1981_Ann.Clin.Biochem_18_9 |
| ESTHER : Whittaker_1981_Ann.Clin.Biochem_18_9 |
| PubMedSearch : Whittaker_1981_Ann.Clin.Biochem_18_9 |
| PubMedID: 7259066 |
| Title : Comparison of atypical and usual human serum cholinesterase. Purification, number of active sites, substrate affinity, and turnover number - Lockridge_1978_J.Biol.Chem_253_361 |
| Author(s) : Lockridge O , La Du BN |
| Ref : Journal of Biological Chemistry , 253 :361 , 1978 |
| Abstract : Lockridge_1978_J.Biol.Chem_253_361 |
| ESTHER : Lockridge_1978_J.Biol.Chem_253_361 |
| PubMedSearch : Lockridge_1978_J.Biol.Chem_253_361 |
| PubMedID: 618874 |