Enalapril
General
Type : Not A\/B H target || Drug || Pro-Drug || Pyrrolidine || Angiotensin-converting enzyme inhibitor
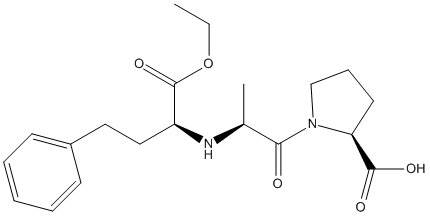
Chemical_Nomenclature : (2S)-1-[(2S)-2-[[(2S)-1-ethoxy-1-oxo-4-phenylbutan-2-yl]amino]propanoyl]pyrrolidine-2-carboxylic acid
Canonical SMILES : CCOC(=O)C(CCC1=CC=CC=C1)NC(C)C(=O)N2CCCC2C(=O)O
InChI : InChI=1S\/C20H28N2O5\/c1-3-27-20(26)16(12-11-15-8-5-4-6-9-15)21-14(2)18(23)22-13-7-10-17(22)19(24)25\/h4-6,8-9,14,16-17,21H,3,7,10-13H2,1-2H3,(H,24,25)\/t14-,16-,17-\/m0\/s1
InChIKey : GBXSMTUPTTWBMN-XIRDDKMYSA-N
Other name(s) : Enalaprilum, Vasotec, Enalaprila, Epaned
Target
Families : Carb_B_Chordata
References (9)
| Title : Pharmacogenetic study of CES1 gene and enalapril efficacy - Hussain_2024_J.Appl.Genet__ |
| Author(s) : Hussain M , Basheer S , Khalil A , Haider QUA , Saeed H , Faizan M |
| Ref : J Appl Genet , : , 2024 |
| Abstract : Hussain_2024_J.Appl.Genet__ |
| ESTHER : Hussain_2024_J.Appl.Genet__ |
| PubMedSearch : Hussain_2024_J.Appl.Genet__ |
| PubMedID: 38261266 |
| Gene_locus related to this paper: human-CES1 |
| Title : The Influence of the CES1 Genotype on the Pharmacokinetics of Enalapril in Patients with Arterial Hypertension - Ikonnikova_2022_J.Pers.Med_12_580 |
| Author(s) : Ikonnikova A , Rodina T , Dmitriev A , Melnikov E , Kazakov R , Nasedkina T |
| Ref : J Pers Med , 12 : , 2022 |
| Abstract : Ikonnikova_2022_J.Pers.Med_12_580 |
| ESTHER : Ikonnikova_2022_J.Pers.Med_12_580 |
| PubMedSearch : Ikonnikova_2022_J.Pers.Med_12_580 |
| PubMedID: 35455696 |
| Gene_locus related to this paper: human-CES1 |
| Title : CES1 genetic variation affects the activation of angiotensin-converting enzyme inhibitors - Wang_2016_Pharmacogenomics.J_16_220 |
| Author(s) : Wang X , Wang G , Shi J , Aa JY , Comas R , Liang Y , Zhu HJ |
| Ref : Pharmacogenomics J , 16 :220 , 2016 |
| Abstract : Wang_2016_Pharmacogenomics.J_16_220 |
| ESTHER : Wang_2016_Pharmacogenomics.J_16_220 |
| PubMedSearch : Wang_2016_Pharmacogenomics.J_16_220 |
| PubMedID: 26076923 |
| Gene_locus related to this paper: human-CES1 |
| Title : Absorption and cleavage of enalapril, a carboxyl ester prodrug, in the rat intestine: In vitro, in situ intestinal perfusion, and portal vein cannulation models - Holenarsipur_2015_Biopharm.Drug.Dispos_36_385 |
| Author(s) : Holenarsipur VK , Gaud N , Sinha J , Sivaprasad S , Bhutani P , Subramanian M , Singh SP , Arla R , Paruchury S , Sharma T , Marathe P , Mandlekar S |
| Ref : Biopharmaceutics & Drug Disposition , 36 :385 , 2015 |
| Abstract : Holenarsipur_2015_Biopharm.Drug.Dispos_36_385 |
| ESTHER : Holenarsipur_2015_Biopharm.Drug.Dispos_36_385 |
| PubMedSearch : Holenarsipur_2015_Biopharm.Drug.Dispos_36_385 |
| PubMedID: 25832562 |
| Title : Effect of carboxylesterase 1 c.428G >\; A single nucleotide variation on the pharmacokinetics of quinapril and enalapril - Tarkiainen_2015_Br.J.Clin.Pharmacol_80_1131 |
| Author(s) : Tarkiainen EK , Tornio A , Holmberg MT , Launiainen T , Neuvonen PJ , Backman JT , Niemi M |
| Ref : British Journal of Clinical Pharmacology , 80 :1131 , 2015 |
| Abstract : Tarkiainen_2015_Br.J.Clin.Pharmacol_80_1131 |
| ESTHER : Tarkiainen_2015_Br.J.Clin.Pharmacol_80_1131 |
| PubMedSearch : Tarkiainen_2015_Br.J.Clin.Pharmacol_80_1131 |
| PubMedID: 25919042 |
| Gene_locus related to this paper: human-CES1 |
| Title : In vitro drug metabolism by human carboxylesterase 1: focus on Angiotensin-converting enzyme inhibitors - Thomsen_2014_Drug.Metab.Dispos_42_126 |
| Author(s) : Thomsen R , Rasmussen HB , Linnet K |
| Ref : Drug Metabolism & Disposition: The Biological Fate of Chemicals , 42 :126 , 2014 |
| Abstract : Thomsen_2014_Drug.Metab.Dispos_42_126 |
| ESTHER : Thomsen_2014_Drug.Metab.Dispos_42_126 |
| PubMedSearch : Thomsen_2014_Drug.Metab.Dispos_42_126 |
| PubMedID: 24141856 |
| Gene_locus related to this paper: human-CES1 |
| Title : Effect of losartan and enalapril on cognitive deficit caused by Goldblatt induced hypertension - Srinivasan_2005_Indian.J.Exp.Biol_43_241 |
| Author(s) : Srinivasan J , Jayadev S , Kumaran D , Ahamed KF , Suresh B , Ramanathan M |
| Ref : Indian J Exp Biol , 43 :241 , 2005 |
| Abstract : Srinivasan_2005_Indian.J.Exp.Biol_43_241 |
| ESTHER : Srinivasan_2005_Indian.J.Exp.Biol_43_241 |
| PubMedSearch : Srinivasan_2005_Indian.J.Exp.Biol_43_241 |
| PubMedID: 15816410 |
| Title : Oculohypotensive effect of angiotensin-converting enzyme inhibitors in acute and chronic models of glaucoma - Shah_2000_J.Cardiovasc.Pharmacol_36_169 |
| Author(s) : Shah GB , Sharma S , Mehta AA , Goyal RK |
| Ref : J Cardiovasc Pharmacol , 36 :169 , 2000 |
| Abstract : Shah_2000_J.Cardiovasc.Pharmacol_36_169 |
| ESTHER : Shah_2000_J.Cardiovasc.Pharmacol_36_169 |
| PubMedSearch : Shah_2000_J.Cardiovasc.Pharmacol_36_169 |
| PubMedID: 10942157 |
| Title : Comparative and combined efficacy of doxazosin and enalapril in hypertensive patients - Stokes_1994_Clin.Exp.Hypertens_16_709 |
| Author(s) : Stokes GS , Johnston HJ , Okoro EO , Boutagy J , Monaghan JC , Marwood JF |
| Ref : Clinical & Experimental Hypertension , 16 :709 , 1994 |
| Abstract : Stokes_1994_Clin.Exp.Hypertens_16_709 |
| ESTHER : Stokes_1994_Clin.Exp.Hypertens_16_709 |
| PubMedSearch : Stokes_1994_Clin.Exp.Hypertens_16_709 |
| PubMedID: 7858555 |