Ethyl-acetoacetate
General
Type : Alkyl ester || Oxobutanoate
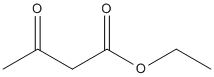
Chemical_Nomenclature : ethyl 3-oxobutanoate
Canonical SMILES : CCOC(=O)CC(=O)C
InChI : InChI=1S\/C6H10O3\/c1-3-9-6(8)4-5(2)7\/h3-4H2,1-2H3
InChIKey : XYIBRDXRRQCHLP-UHFFFAOYSA-N
Other name(s) : Ethyl acetoacetate, Ethyl 3-oxobutanoate, Ethyl acetylacetate, Ethyl 3-oxobutyrate, Diacetic ether
References (4)
| Title : PPL catalyzed four-component PASE synthesis of 5-monosubstituted barbiturates: Structure and pharmacological properties - Bihani_2015_Bioorg.Med.Chem.Lett_25_5732 |
| Author(s) : Bihani M , Bora PP , Verma AK , Baruah R , Boruah HP , Bez G |
| Ref : Bioorganic & Medicinal Chemistry Lett , 25 :5732 , 2015 |
| Abstract : Bihani_2015_Bioorg.Med.Chem.Lett_25_5732 |
| ESTHER : Bihani_2015_Bioorg.Med.Chem.Lett_25_5732 |
| PubMedSearch : Bihani_2015_Bioorg.Med.Chem.Lett_25_5732 |
| PubMedID: 26546212 |
| Title : Hyperpolarized [1,3-13C2 ]ethyl acetoacetate is a novel diagnostic metabolic marker of liver cancer - Jensen_2015_Int.J.Cancer_136_E117 |
| Author(s) : Jensen PR , Serra SC , Miragoli L , Karlsson M , Cabella C , Poggi L , Venturi L , Tedoldi F , Lerche MH |
| Ref : International Journal of Cancer , 136 :E117 , 2015 |
| Abstract : Jensen_2015_Int.J.Cancer_136_E117 |
| ESTHER : Jensen_2015_Int.J.Cancer_136_E117 |
| PubMedSearch : Jensen_2015_Int.J.Cancer_136_E117 |
| PubMedID: 25156718 |
| Title : A novel extracellular esterase from Bacillus subtilis and its conversion to a monoacylglycerol hydrolase - Eggert_2000_Eur.J.Biochem_267_6459 |
| Author(s) : Eggert T , Pencreac'h G , Douchet I , Verger R , Jaeger KE |
| Ref : European Journal of Biochemistry , 267 :6459 , 2000 |
| Abstract : Eggert_2000_Eur.J.Biochem_267_6459 |
| ESTHER : Eggert_2000_Eur.J.Biochem_267_6459 |
| PubMedSearch : Eggert_2000_Eur.J.Biochem_267_6459 |
| PubMedID: 11029590 |
| Gene_locus related to this paper: bacsu-LIPB |