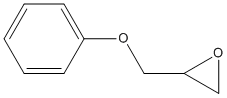
Glycidyl-phenyl-ether
General
Type : Epoxide
Chemical_Nomenclature : 2-(phenoxymethyl)oxirane
Canonical SMILES : C1C(O1)COC2=CC=CC=C2
InChI : InChI=1S\/C9H10O2\/c1-2-4-8(5-3-1)10-6-9-7-11-9\/h1-5,9H,6-7H2
InChIKey : FQYUMYWMJTYZTK-UHFFFAOYSA-N
Other name(s) : glycidyl phenyl ether, 2-(Phenoxymethyl)oxirane, Phenyl glycidyl ether, 1,2-Epoxy-3-phenoxypropane, Oxirane, (phenoxymethyl)-
Target
Families : Epoxide_hydrolase
References (10)
| Title : Enantioselective Hydrolysis of Styrene Oxide and Benzyl Glycidyl Ether by a Variant of Epoxide Hydrolase from Agromyces mediolanus - Jin_2019_Mar.Drugs_17_ |
| Author(s) : Jin H , Li Y , Zhang Q , Lin S , Yang Z , Ding G |
| Ref : Mar Drugs , 17 : , 2019 |
| Abstract : Jin_2019_Mar.Drugs_17_ |
| ESTHER : Jin_2019_Mar.Drugs_17_ |
| PubMedSearch : Jin_2019_Mar.Drugs_17_ |
| PubMedID: 31226863 |
| Gene_locus related to this paper: agrme-a0a088b180 |
| Title : Effect of Binding on Enantioselectivity of Epoxide Hydrolase - Zaugg_2018_J.Chem.Inf.Model_58_630 |
| Author(s) : Zaugg J , Gumulya Y , Boden M , Mark AE , Malde AK |
| Ref : J Chem Inf Model , 58 :630 , 2018 |
| Abstract : Zaugg_2018_J.Chem.Inf.Model_58_630 |
| ESTHER : Zaugg_2018_J.Chem.Inf.Model_58_630 |
| PubMedSearch : Zaugg_2018_J.Chem.Inf.Model_58_630 |
| PubMedID: 29424533 |
| Gene_locus related to this paper: aspni-hyl1 |
| Title : Bioresolution of racemic phenyl glycidyl ether by a putative recombinant epoxide hydrolase from Streptomyces griseus NBRC 13350 - Saini_2017_World.J.Microbiol.Biotechnol_33_82 |
| Author(s) : Saini P , Kumar N , Wani SI , Sharma S , Chimni SS , Sareen D |
| Ref : World J Microbiol Biotechnol , 33 :82 , 2017 |
| Abstract : Saini_2017_World.J.Microbiol.Biotechnol_33_82 |
| ESTHER : Saini_2017_World.J.Microbiol.Biotechnol_33_82 |
| PubMedSearch : Saini_2017_World.J.Microbiol.Biotechnol_33_82 |
| PubMedID: 28378221 |
| Gene_locus related to this paper: strgg-b1vn62 |
| Title : Effect of fungal mycelia on the HPLC-UV and UV-vis spectrophotometric assessment of mycelium-bound epoxide hydrolase using glycidyl phenyl ether - Dolcet_2016_N.Biotechnol_33_449 |
| Author(s) : Dolcet MM , Torres M , Canela R |
| Ref : N Biotechnol , 33 :449 , 2016 |
| Abstract : Dolcet_2016_N.Biotechnol_33_449 |
| ESTHER : Dolcet_2016_N.Biotechnol_33_449 |
| PubMedSearch : Dolcet_2016_N.Biotechnol_33_449 |
| PubMedID: 26902669 |
| Title : Efficient kinetic resolution of phenyl glycidyl ether by a novel epoxide hydrolase from Tsukamurella paurometabola - Wu_2015_Appl.Microbiol.Biotechnol_99_9511 |
| Author(s) : Wu K , Wang H , Sun H , Wei D |
| Ref : Applied Microbiology & Biotechnology , 99 :9511 , 2015 |
| Abstract : Wu_2015_Appl.Microbiol.Biotechnol_99_9511 |
| ESTHER : Wu_2015_Appl.Microbiol.Biotechnol_99_9511 |
| PubMedSearch : Wu_2015_Appl.Microbiol.Biotechnol_99_9511 |
| PubMedID: 26088175 |
| Gene_locus related to this paper: tsupd-d5us69 |
| Title : Biocatalytic resolution of glycidyl phenyl ether using a novel epoxide hydrolase from a marine bacterium, Maritimibacter alkaliphilus KCCM 42376 [corrected] - Woo_2010_J.Biosci.Bioeng_109_539 |
| Author(s) : Woo JH , Kang JH , Hwang YO , Cho JC , Kim SJ , Kang SG |
| Ref : J Biosci Bioeng , 109 :539 , 2010 |
| Abstract : Woo_2010_J.Biosci.Bioeng_109_539 |
| ESTHER : Woo_2010_J.Biosci.Bioeng_109_539 |
| PubMedSearch : Woo_2010_J.Biosci.Bioeng_109_539 |
| PubMedID: 20471590 |
| Gene_locus related to this paper: 9rhob-a3vif4 |
| Title : Directed evolution of an enantioselective epoxide hydrolase: uncovering the source of enantioselectivity at each evolutionary stage - Reetz_2009_J.Am.Chem.Soc_131_7334 |
| Author(s) : Reetz MT , Bocola M , Wang LW , Sanchis J , Cronin A , Arand M , Zou J , Archelas A , Bottalla AL , Naworyta A , Mowbray SL |
| Ref : Journal of the American Chemical Society , 131 :7334 , 2009 |
| Abstract : Reetz_2009_J.Am.Chem.Soc_131_7334 |
| ESTHER : Reetz_2009_J.Am.Chem.Soc_131_7334 |
| PubMedSearch : Reetz_2009_J.Am.Chem.Soc_131_7334 |
| PubMedID: 19469578 |
| Gene_locus related to this paper: aspni-hyl1 |
| Title : A cold-adapted epoxide hydrolase from a strict marine bacterium, Sphingophyxis alaskensis - Kang_2008_J.Microbiol.Biotechnol_18_1445 |
| Author(s) : Kang JH , Woo JH , Kang SG , Hwang YO , Kim SJ |
| Ref : J Microbiol Biotechnol , 18 :1445 , 2008 |
| Abstract : Kang_2008_J.Microbiol.Biotechnol_18_1445 |
| ESTHER : Kang_2008_J.Microbiol.Biotechnol_18_1445 |
| PubMedSearch : Kang_2008_J.Microbiol.Biotechnol_18_1445 |
| PubMedID: 18756107 |
| Gene_locus related to this paper: 9sphn-q3vax0 |
| Title : A novel enantioselective epoxide hydrolase for (R)-phenyl glycidyl ether to generate (R)-3-phenoxy-1,2-propanediol - Wu_2007_Appl.Microbiol.Biotechnol_76_1281 |
| Author(s) : Wu S , Shen J , Zhou X , Chen J |
| Ref : Applied Microbiology & Biotechnology , 76 :1281 , 2007 |
| Abstract : Wu_2007_Appl.Microbiol.Biotechnol_76_1281 |
| ESTHER : Wu_2007_Appl.Microbiol.Biotechnol_76_1281 |
| PubMedSearch : Wu_2007_Appl.Microbiol.Biotechnol_76_1281 |
| PubMedID: 17710393 |
| Gene_locus related to this paper: tsupd-d5us69 |
| Title : Enhancing the enantioselectivity of an epoxide hydrolase by directed evolution - Reetz_2004_Org.Lett_6_177 |
| Author(s) : Reetz MT , Torre C , Eipper A , Lohmer R , Hermes M , Brunner B , Maichele A , Bocola M , Arand M , Cronin A , Genzel Y , Archelas A , Furstoss R |
| Ref : Org Lett , 6 :177 , 2004 |
| Abstract : Reetz_2004_Org.Lett_6_177 |
| ESTHER : Reetz_2004_Org.Lett_6_177 |
| PubMedSearch : Reetz_2004_Org.Lett_6_177 |
| PubMedID: 14723522 |
| Gene_locus related to this paper: aspni-hyl1 |