HODA-6-methyl
General
Type : Meta-Cleavage-Product
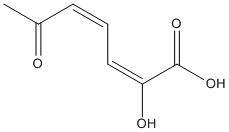
Chemical_Nomenclature : (2E,4Z)-2-hydroxy-6-oxohepta-2,4-dienoic acid
Canonical SMILES : CC(=O)C=CC=C(C(=O)O)O
InChI : InChI=1S\/C7H8O4\/c1-5(8)3-2-4-6(9)7(10)11\/h2-4,9H,1H3,(H,10,11)\/b3-2-,6-4+
InChIKey : HVZGWILTESYJSP-MGLVMYNNSA-N
Other name(s) : 2-hydroxy-6-oxohepta-2,4-dienoate, 2-Hydroxy-6-keto-2,4-heptadienoate, 2-Hydroxy-6-oxo-hept-2,4-dienoate, C06210, CHEBI:1132, SCHEMBL2931476, (2E,4Z)-2-hydroxy-6-oxo-hepta-2,4-dienoic acid, 6-methyl-HODA, HOHD
MW : 156.13
Formula : C7H8O4
CAS_number :
PubChem :
UniChem :
Iuphar :
Target
Families : Carbon-carbon_bond_hydrolase, BD-FAE
References (2)
| Title : A novel meta-cleavage product hydrolase from Flavobacterium sp. ATCC27551 - Khajamohiddin_2006_Biochem.Biophys.Res.Commun_351_675 |
| Author(s) : Khajamohiddin S , Babu PS , Chakka D , Merrick M , Bhaduri A , Sowdhamini R , Siddavattam D |
| Ref : Biochemical & Biophysical Research Communications , 351 :675 , 2006 |
| Abstract : Khajamohiddin_2006_Biochem.Biophys.Res.Commun_351_675 |
| ESTHER : Khajamohiddin_2006_Biochem.Biophys.Res.Commun_351_675 |
| PubMedSearch : Khajamohiddin_2006_Biochem.Biophys.Res.Commun_351_675 |
| PubMedID: 17078928 |
| Gene_locus related to this paper: sphsa-q8gc44 |
| Title : A series of crystal structures of a meta-cleavage product hydrolase from Pseudomonas fluorescens IP01 (CumD) complexed with various cleavage products - Fushinobu_2005_Biosci.Biotechnol.Biochem_69_491 |
| Author(s) : Fushinobu S , Jun SY , Hidaka M , Nojiri H , Yamane H , Shoun H , Omori T , Wakagi T |
| Ref : Biosci Biotechnol Biochem , 69 :491 , 2005 |
| Abstract : Fushinobu_2005_Biosci.Biotechnol.Biochem_69_491 |
| ESTHER : Fushinobu_2005_Biosci.Biotechnol.Biochem_69_491 |
| PubMedSearch : Fushinobu_2005_Biosci.Biotechnol.Biochem_69_491 |
| PubMedID: 15784976 |
| Gene_locus related to this paper: psefl-cumD |