Heliolactone
General
Type : Butenolide || Strigolactone receptors ligand
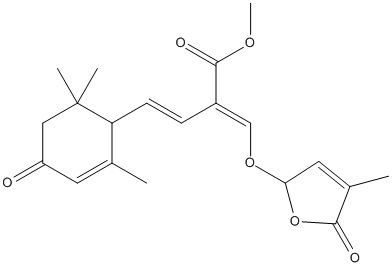
Chemical_Nomenclature : methyl (E,2E)-2-[(4-methyl-5-oxo-2H-furan-2-yl)oxymethylidene]-4-(2,6,6-trimethyl-4-oxocyclohex-2-en-1-yl)but-3-enoate
Canonical SMILES : CC1=CC(OC1=O)OC=C(C=CC2C(=CC(=O)CC2(C)C)C)C(=O)OC
InChI : InChI=1S\/C20H24O6\/c1-12-8-15(21)10-20(3,4)16(12)7-6-14(19(23)24-5)11-25-17-9-13(2)18(22)26-17\/h6-9,11,16-17H,10H2,1-5H3\/b7-6+,14-11+
InChIKey : VPSDSSLYHQLBLR-WXGDJGGUSA-N
Other name(s) :
MW : 360.4
Formula : C20H24O6
CAS_number :
PubChem :
UniChem :
Iuphar :
Target
Families : RsbQ-like
References (5)
| Title : Identification of 6-epi-heliolactone as a biosynthetic precursor of avenaol in Avena strigosa - Moriyama_2022_Biosci.Biotechnol.Biochem_86_998 |
| Author(s) : Moriyama D , Wakabayashi T , Shiotani N , Yamamoto S , Furusato Y , Yabe K , Mizutani M , Takikawa H , Sugimoto Y |
| Ref : Biosci Biotechnol Biochem , 86 :998 , 2022 |
| Abstract : Moriyama_2022_Biosci.Biotechnol.Biochem_86_998 |
| ESTHER : Moriyama_2022_Biosci.Biotechnol.Biochem_86_998 |
| PubMedSearch : Moriyama_2022_Biosci.Biotechnol.Biochem_86_998 |
| PubMedID: 35561745 |
| Title : Conversion of methyl carlactonoate to heliolactone in sunflower - Wakabayashi_2022_Nat.Prod.Res_36_2215 |
| Author(s) : Wakabayashi T , Shinde H , Shiotani N , Yamamoto S , Mizutani M , Takikawa H , Sugimoto Y |
| Ref : Nat Prod Res , 36 :2215 , 2022 |
| Abstract : Wakabayashi_2022_Nat.Prod.Res_36_2215 |
| ESTHER : Wakabayashi_2022_Nat.Prod.Res_36_2215 |
| PubMedSearch : Wakabayashi_2022_Nat.Prod.Res_36_2215 |
| PubMedID: 33034235 |
| Title : Concise synthesis of heliolactone, a non-canonical strigolactone isolated from sunflower - Yamamoto_2020_Biosci.Biotechnol.Biochem_84_1113 |
| Author(s) : Yamamoto S , Atarashi T , Kuse M , Sugimoto Y , Takikawa H |
| Ref : Biosci Biotechnol Biochem , 84 :1113 , 2020 |
| Abstract : Yamamoto_2020_Biosci.Biotechnol.Biochem_84_1113 |
| ESTHER : Yamamoto_2020_Biosci.Biotechnol.Biochem_84_1113 |
| PubMedSearch : Yamamoto_2020_Biosci.Biotechnol.Biochem_84_1113 |
| PubMedID: 32116121 |
| Title : Lotuslactone, a non-canonical strigolactone from Lotus japonicus - Xie_2019_Phytochemistry_157_200 |
| Author(s) : Xie X , Mori N , Yoneyama K , Nomura T , Uchida K , Akiyama K |
| Ref : Phytochemistry , 157 :200 , 2019 |
| Abstract : Xie_2019_Phytochemistry_157_200 |
| ESTHER : Xie_2019_Phytochemistry_157_200 |
| PubMedSearch : Xie_2019_Phytochemistry_157_200 |
| PubMedID: 30439621 |
| Title : Heliolactone, a non-sesquiterpene lactone germination stimulant for root parasitic weeds from sunflower - Ueno_2014_Phytochemistry_108_122 |
| Author(s) : Ueno K , Furumoto T , Umeda S , Mizutani M , Takikawa H , Batchvarova R , Sugimoto Y |
| Ref : Phytochemistry , 108 :122 , 2014 |
| Abstract : Ueno_2014_Phytochemistry_108_122 |
| ESTHER : Ueno_2014_Phytochemistry_108_122 |
| PubMedSearch : Ueno_2014_Phytochemistry_108_122 |
| PubMedID: 25446236 |