Irinotecan
General
Type : Carbamate || Drug || Derivative of Irinotecan
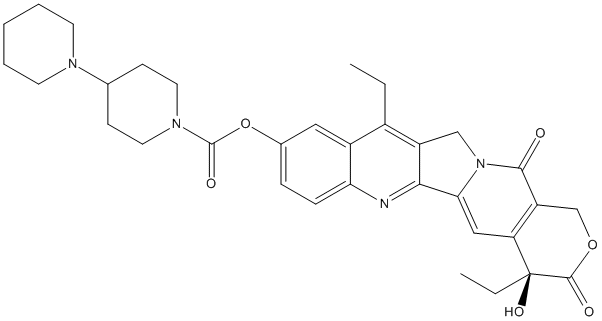
Chemical_Nomenclature : 7-ethyl-10-[4-(1-piperidino)-1-piperidino]carbonyloxycamptothecin)
Canonical SMILES : CCC1=C2C=C(C=CC2=NC3=C1CN4C3=CC5=C(C4=O)COC(=O)C5(CC)O)OC(=O)N6CCC(CC6)N7CCCCC7
InChI : InChI=1S\/C33H38N4O6\/c1-3-22-23-16-21(43-32(40)36-14-10-20(11-15-36)35-12-6-5-7-13-35)8-9-27(23)34-29-24(22)18-37-28(29)17-26-25(30(37)38)19-42-31(39)33(26,41)4-2\/h8-9,16-17,20,41H,3-7,10-15,18-19H2,1-2H3\/t33-\/m0\/s1
InChIKey : UWKQSNNFCGGAFS-XIFFEERXSA-N
Other name(s) : Camptosar, Irinotecanum, Biotecan, Topotecin, Campto, Irinotecan Hcl, CPT 11, CPT11
Target
Families : Carb_B_Chordata, BCHE, Carb_B_Bacteria
References (18)
| Title : Structural insights into catalytical capability for CPT11 hydrolysis and substrate specificity of a novel marine microbial carboxylesterase, E93 - Li_2023_Front.Microbiol_13_1081094 |
| Author(s) : Li Y , Rong Z , Li Z , Cui H , Li J , Xu XW |
| Ref : Front Microbiol , 13 :1081094 , 2023 |
| Abstract : Li_2023_Front.Microbiol_13_1081094 |
| ESTHER : Li_2023_Front.Microbiol_13_1081094 |
| PubMedSearch : Li_2023_Front.Microbiol_13_1081094 |
| PubMedID: 36756200 |
| Gene_locus related to this paper: 9sphn-E93 |
| Title : In Vitro Evaluation of the Inhibitory Potential of Pharmaceutical Excipients on Human Carboxylesterase 1A and 2 - Zhang_2014_PLoS.One_9_e93819 |
| Author(s) : Zhang C , Xu Y , Zhong Q , Li X , Gao P , Feng C , Chu Q , Chen Y , Liu D |
| Ref : PLoS ONE , 9 :e93819 , 2014 |
| Abstract : Zhang_2014_PLoS.One_9_e93819 |
| ESTHER : Zhang_2014_PLoS.One_9_e93819 |
| PubMedSearch : Zhang_2014_PLoS.One_9_e93819 |
| PubMedID: 24699684 |
| Title : Mammalian carboxylesterases: from drug targets to protein therapeutics - Redinbo_2005_Drug.Discov.Today_10_313 |
| Author(s) : Redinbo MR , Potter PM |
| Ref : Drug Discov Today , 10 :313 , 2005 |
| Abstract : Redinbo_2005_Drug.Discov.Today_10_313 |
| ESTHER : Redinbo_2005_Drug.Discov.Today_10_313 |
| PubMedSearch : Redinbo_2005_Drug.Discov.Today_10_313 |
| PubMedID: 15749280 |
| Title : Lessons learned from the irinotecan metabolic pathway - Ma_2003_Curr.Med.Chem_10_41 |
| Author(s) : Ma MK , McLeod HL |
| Ref : Curr Med Chem , 10 :41 , 2003 |
| Abstract : Ma_2003_Curr.Med.Chem_10_41 |
| ESTHER : Ma_2003_Curr.Med.Chem_10_41 |
| PubMedSearch : Ma_2003_Curr.Med.Chem_10_41 |
| PubMedID: 12570720 |
| Title : Structural insights into CPT-11 activation by mammalian carboxylesterases - Bencharit_2002_Nat.Struct.Biol_9_337 |
| Author(s) : Bencharit S , Morton CL , Howard-Williams EL , Danks MK , Potter PM , Redinbo MR |
| Ref : Nat Struct Biol , 9 :337 , 2002 |
| Abstract : Bencharit_2002_Nat.Struct.Biol_9_337 |
| ESTHER : Bencharit_2002_Nat.Struct.Biol_9_337 |
| PubMedSearch : Bencharit_2002_Nat.Struct.Biol_9_337 |
| PubMedID: 11967565 |
| Gene_locus related to this paper: rabit-1cxes |
| Title : A new metabolite of irinotecan in which formation is mediated by human hepatic cytochrome P-450 3A4 - Sai_2001_Drug.Metab.Dispos_29_1505 |
| Author(s) : Sai K , Kaniwa N , Ozawa S , Sawada JI |
| Ref : Drug Metabolism & Disposition: The Biological Fate of Chemicals , 29 :1505 , 2001 |
| Abstract : Sai_2001_Drug.Metab.Dispos_29_1505 |
| ESTHER : Sai_2001_Drug.Metab.Dispos_29_1505 |
| PubMedSearch : Sai_2001_Drug.Metab.Dispos_29_1505 |
| PubMedID: 11602529 |
| Title : Isolation and characterization of a cDNA encoding a horse liver butyrylcholinesterase: evidence for CPT-11 drug activation - Wierdl_2000_Biochem.Pharmacol_59_773 |
| Author(s) : Wierdl M , Morton CL , Danks MK , Potter PM |
| Ref : Biochemical Pharmacology , 59 :773 , 2000 |
| Abstract : Wierdl_2000_Biochem.Pharmacol_59_773 |
| ESTHER : Wierdl_2000_Biochem.Pharmacol_59_773 |
| PubMedSearch : Wierdl_2000_Biochem.Pharmacol_59_773 |
| PubMedID: 10718335 |
| Gene_locus related to this paper: horse-BCHE |
| Title : The anticancer prodrug CPT-11 is a potent inhibitor of acetylcholinesterase but is rapidly catalyzed to SN-38 by butyrylcholinesterase - Morton_1999_Cancer.Res_59_1458 |
| Author(s) : Morton CL , Wadkins RM , Danks MK , Potter PM |
| Ref : Cancer Research , 59 :1458 , 1999 |
| Abstract : Morton_1999_Cancer.Res_59_1458 |
| ESTHER : Morton_1999_Cancer.Res_59_1458 |
| PubMedSearch : Morton_1999_Cancer.Res_59_1458 |
| PubMedID: 10197614 |
| Title : The mechanism for the inhibition of acetylcholinesterases by irinotecan (CPT-11) - Dodds_1999_Mol.Pharmacol_56_1346 |
| Author(s) : Dodds HM , Rivory LP |
| Ref : Molecular Pharmacology , 56 :1346 , 1999 |
| Abstract : Dodds_1999_Mol.Pharmacol_56_1346 |
| ESTHER : Dodds_1999_Mol.Pharmacol_56_1346 |
| PubMedSearch : Dodds_1999_Mol.Pharmacol_56_1346 |
| PubMedID: 10570064 |
| Title : Water soluble 20(S)-glycinate esters of 10,11-methylenedioxycamptothecins are highly active against human breast cancer xenografts - Wadkins_1999_Cancer.Res_59_3424 |
| Author(s) : Wadkins RM , Potter PM , Vladu B , Marty J , Mangold G , Weitman S , Manikumar G , Wani MC , Wall ME , Von Hoff DD |
| Ref : Cancer Research , 59 :3424 , 1999 |
| Abstract : Wadkins_1999_Cancer.Res_59_3424 |
| ESTHER : Wadkins_1999_Cancer.Res_59_3424 |
| PubMedSearch : Wadkins_1999_Cancer.Res_59_3424 |
| PubMedID: 10416605 |
| Title : Conversion of the CPT-11 metabolite APC to SN-38 by rabbit liver carboxylesterase - Guichard_1998_Clin.Cancer.Res_4_3089 |
| Author(s) : Guichard SM , Morton CL , Krull EJ , Stewart CF , Danks MK , Potter PM |
| Ref : Clin Cancer Research , 4 :3089 , 1998 |
| Abstract : Guichard_1998_Clin.Cancer.Res_4_3089 |
| ESTHER : Guichard_1998_Clin.Cancer.Res_4_3089 |
| PubMedSearch : Guichard_1998_Clin.Cancer.Res_4_3089 |
| PubMedID: 9865925 |
| Title : Gastrointestinal toxicity or irinotecan - Hecht_1998_Oncology_12_72 |
| Author(s) : Hecht JR |
| Ref : Oncology , 12 :72 , 1998 |
| Abstract : Hecht_1998_Oncology_12_72 |
| ESTHER : Hecht_1998_Oncology_12_72 |
| PubMedSearch : Hecht_1998_Oncology_12_72 |
| PubMedID: 9726096 |
| Title : Pharmacology of irinotecan - Kuhn_1998_Oncology_12_39 |
| Author(s) : Kuhn JG |
| Ref : Oncology , 12 :39 , 1998 |
| Abstract : Kuhn_1998_Oncology_12_39 |
| ESTHER : Kuhn_1998_Oncology_12_39 |
| PubMedSearch : Kuhn_1998_Oncology_12_39 |
| PubMedID: 9726089 |
| Title : Cellular localization domains of a rabbit and a human carboxylesterase: influence on irinotecan (CPT-11) metabolism by the rabbit enzyme - Potter_1998_Cancer.Res_58_3627 |
| Author(s) : Potter PM , Wolverton JS , Morton CL , Wierdl M , Danks MK |
| Ref : Cancer Research , 58 :3627 , 1998 |
| Abstract : Potter_1998_Cancer.Res_58_3627 |
| ESTHER : Potter_1998_Cancer.Res_58_3627 |
| PubMedSearch : Potter_1998_Cancer.Res_58_3627 |
| PubMedID: 9721871 |
| Title : Overexpression of a rabbit liver carboxylesterase sensitizes human tumor cells to CPT-11 - Danks_1998_Cancer.Res_58_20 |
| Author(s) : Danks MK , Morton CL , Pawlik CA , Potter PM |
| Ref : Cancer Research , 58 :20 , 1998 |
| Abstract : Danks_1998_Cancer.Res_58_20 |
| ESTHER : Danks_1998_Cancer.Res_58_20 |
| PubMedSearch : Danks_1998_Cancer.Res_58_20 |
| PubMedID: 9426050 |
| Gene_locus related to this paper: rabit-1cxes |
| Title : Isolation and partial characterization of a cDNA encoding a rabbit liver carboxylesterase that activates the prodrug irinotecan (CPT-11) - Potter_1998_Cancer.Res_58_2646 |
| Author(s) : Potter PM , Pawlik CA , Morton CL , Naeve CW , Danks MK |
| Ref : Cancer Research , 58 :2646 , 1998 |
| Abstract : Potter_1998_Cancer.Res_58_2646 |
| ESTHER : Potter_1998_Cancer.Res_58_2646 |
| PubMedSearch : Potter_1998_Cancer.Res_58_2646 |
| PubMedID: 9635592 |
| Gene_locus related to this paper: rabit-1cxes |
| Title : CPT-11 in human colon-cancer cell lines and xenografts: characterization of cellular sensitivity determinants - Jansen_1997_Int.J.Cancer_70_335 |
| Author(s) : Jansen WJ , Zwart B , Hulscher ST , Giaccone G , Pinedo HM , Boven E |
| Ref : International Journal of Cancer , 70 :335 , 1997 |
| Abstract : Jansen_1997_Int.J.Cancer_70_335 |
| ESTHER : Jansen_1997_Int.J.Cancer_70_335 |
| PubMedSearch : Jansen_1997_Int.J.Cancer_70_335 |
| PubMedID: 9033637 |
| Title : Identification and properties of a major plasma metabolite of irinotecan (CPT-11) isolated from the plasma of patients - Rivory_1996_Cancer.Res_56_3689 |
| Author(s) : Rivory LP , Riou JF , Haaz MC , Sable S , Vuilhorgne M , Commercon A , Pond SM , Robert J |
| Ref : Cancer Research , 56 :3689 , 1996 |
| Abstract : Rivory_1996_Cancer.Res_56_3689 |
| ESTHER : Rivory_1996_Cancer.Res_56_3689 |
| PubMedSearch : Rivory_1996_Cancer.Res_56_3689 |
| PubMedID: 8706009 |