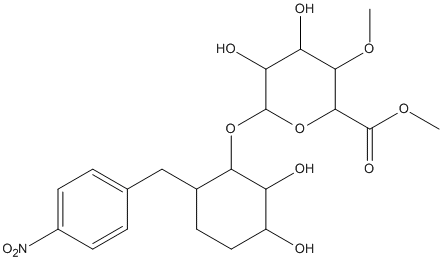
NPh-Xyl-MeGlcA
General
Type : Glucuronoyl || Carbohydrate || Glycoside || pNP
Chemical_Nomenclature : 4-nitrophenyl 2-O-(methyl-4-O-methyl-alpha-D-glucopyranosyluronate)-beta-D-xylopyranoside
Canonical SMILES : C1(C(C(OC(C1OC)C(=O)OC)OC2C(C(CCC2OC3=CC=C(C=C3)[N+](=O)[O-])O)O)O)O
InChI : InChI=1S\/C20H27NO12\/c1-29-17-14(24)15(25)20(33-18(17)19(26)30-2)32-16-12(8-7-11(22)13(16)23)31-10-5-3-9(4-6-10)21(27)28\/h3-6,11-18,20,22-25H,7-8H2,1-2H3
InChIKey : FWSGJYVQHOGSTG-UHFFFAOYSA-N
Other name(s) : pNP-2-O-(methyl-4-O-methyl-alpha-D-glucopyranosyluronate)-D-Xyl
MW : 473.43
Formula : C20H27NO12
CAS_number :
PubChem :
UniChem :
Iuphar :
Target
Families : Glucuronoyl_esterase
References (2)
| Title : Purification, characterization and mass spectrometric sequencing of a thermophilic glucuronoyl esterase from Sporotrichum thermophile. - Vafiadi_2009_FEMS.Microbiol.Lett_296_178 |
| Author(s) : Vafiadi C , Topakas E , Biely P , Christakopoulos P |
| Ref : FEMS Microbiology Letters , 296 :178 , 2009 |
| Abstract : Vafiadi_2009_FEMS.Microbiol.Lett_296_178 |
| ESTHER : Vafiadi_2009_FEMS.Microbiol.Lett_296_178 |
| PubMedSearch : Vafiadi_2009_FEMS.Microbiol.Lett_296_178 |
| PubMedID: 19459957 |
| Gene_locus related to this paper: chagb-Q2GMN0 , thiha-cip2 |
| Title : A chromogenic substrate for a beta-xylosidase-coupled assay of alpha-glucuronidase - Biely_2000_Anal.Biochem_286_289 |
| Author(s) : Biely P , Hirsch J , la Grange DC , van Zyl WH , Prior BA |
| Ref : Analytical Biochemistry , 286 :289 , 2000 |
| Abstract : Biely_2000_Anal.Biochem_286_289 |
| ESTHER : Biely_2000_Anal.Biochem_286_289 |
| PubMedSearch : Biely_2000_Anal.Biochem_286_289 |
| PubMedID: 11067752 |