Paraoxon
General
Type : Organophosphate || Insecticide || Derivative of Paraoxon || pNP
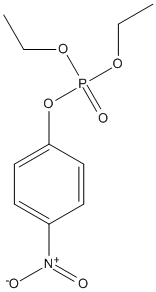
Chemical_Nomenclature : diethyl (4-nitrophenyl) phosphate
Canonical SMILES : CCOP(=O)(OCC)OC1=CC=C(C=C1)[N+](=O)[O-]
InChI : InChI=1S\/C10H14NO6P\/c1-3-15-18(14,16-4-2)17-10-7-5-9(6-8-10)11(12)13\/h5-8H,3-4H2,1-2H3
InChIKey : WYMSBXTXOHUIGT-UHFFFAOYSA-N
Other name(s) : phosphoric acid diethyl 4-nitrophenyl ester, paraoxon-ethyl, Diethyl 4-nitrophenyl phosphate, diethyl p-nitrophenyl phosphate, phosphacol, E600, ester25, eticol, phosphakol, nintacol, miotisal A, solumlaucit, Mintacol, Fosfakol, Ethyl paraoxon, DNP, Diethyl paraoxon, paraoxon ethyl
Target
Families : BCHE, Dienelactone_hydrolase
References (14)
| Title : Ultrahigh-Throughput Directed Evolution of a Metal-Free alpha\/beta-Hydrolase with a Cys-His-Asp Triad into an Efficient Phosphotriesterase - Schnettler_2022_J.Am.Chem.Soc__ |
| Author(s) : Schnettler JD , Klein OJ , Kaminski TS , Colin PY , Hollfelder F |
| Ref : Journal of the American Chemical Society , : , 2022 |
| Abstract : Schnettler_2022_J.Am.Chem.Soc__ |
| ESTHER : Schnettler_2022_J.Am.Chem.Soc__ |
| PubMedSearch : Schnettler_2022_J.Am.Chem.Soc__ |
| PubMedID: 36583539 |
| Gene_locus related to this paper: 9bact-KP212148 |
| Title : Structural and dynamic effects of paraoxon binding to human acetylcholinesterase by X-ray crystallography and inelastic neutron scattering - Gerlits_2022_Structure_30_1538 |
| Author(s) : Gerlits O , Fajer M , Cheng X , Blumenthal DK , Radic Z , Kovalevsky A |
| Ref : Structure , 30 :1538 , 2022 |
| Abstract : Gerlits_2022_Structure_30_1538 |
| ESTHER : Gerlits_2022_Structure_30_1538 |
| PubMedSearch : Gerlits_2022_Structure_30_1538 |
| PubMedID: 36265484 |
| Gene_locus related to this paper: human-ACHE |
| Title : A Thermophilic Bacterial Esterase for Scavenging Nerve Agents: A Kinetic, Biophysical and Structural Study - Bzdrenga_2021_Molecules_26_657 |
| Author(s) : Bzdrenga J , Trenet E , Chantegreil F , Bernal K , Nachon F , Brazzolotto X |
| Ref : Molecules , 26 :657 , 2021 |
| Abstract : Bzdrenga_2021_Molecules_26_657 |
| ESTHER : Bzdrenga_2021_Molecules_26_657 |
| PubMedSearch : Bzdrenga_2021_Molecules_26_657 |
| PubMedID: 33513869 |
| Gene_locus related to this paper: 9bact-TtEst2 |
| Title : Positron emission tomography evaluation of oxime countermeasures in live rats using the tracer O-(2-[(18) F]fluoroethyl)-O-(p-nitrophenyl)methylphosphonate [(18) F]-VXS - Hayes_2020_Ann.N.Y.Acad.Sci__ |
| Author(s) : Hayes TR , Blecha JE , Chao CK , Huynh TL , VanBrocklin HD , Zinn KR , Taylor P , Gerdes JM , Thompson CM |
| Ref : Annals of the New York Academy of Sciences , : , 2020 |
| Abstract : Hayes_2020_Ann.N.Y.Acad.Sci__ |
| ESTHER : Hayes_2020_Ann.N.Y.Acad.Sci__ |
| PubMedSearch : Hayes_2020_Ann.N.Y.Acad.Sci__ |
| PubMedID: 32436233 |
| Title : Crystal Structure of Proteus mirabilis Lipase, a Novel Lipase from the Proteus\/Psychrophilic Subfamily of Lipase Family I.1 - Korman_2012_PLoS.One_7_e52890 |
| Author(s) : Korman TP , Bowie JU |
| Ref : PLoS ONE , 7 :e52890 , 2012 |
| Abstract : Korman_2012_PLoS.One_7_e52890 |
| ESTHER : Korman_2012_PLoS.One_7_e52890 |
| PubMedSearch : Korman_2012_PLoS.One_7_e52890 |
| PubMedID: 23300806 |
| Gene_locus related to this paper: promi-c2lfd0 |
| Title : A role for His-160 in peroxide inhibition of S. cerevisiae S-formylglutathione hydrolase: evidence for an oxidation sensitive motif - Legler_2012_Arch.Biochem.Biophys_528_7 |
| Author(s) : Legler PM , Leary DH , Hervey WJt , Millard CB |
| Ref : Archives of Biochemistry & Biophysics , 528 :7 , 2012 |
| Abstract : Legler_2012_Arch.Biochem.Biophys_528_7 |
| ESTHER : Legler_2012_Arch.Biochem.Biophys_528_7 |
| PubMedSearch : Legler_2012_Arch.Biochem.Biophys_528_7 |
| PubMedID: 22906720 |
| Gene_locus related to this paper: yeast-yjg8 |
| Title : Mutagenesis of organophosphorus hydrolase to enhance hydrolysis of the nerve agent VX - Gopal_2000_Biochem.Biophys.Res.Commun_279_516 |
| Author(s) : Gopal S , Rastogi V , Ashman W , Mulbry W |
| Ref : Biochemical & Biophysical Research Communications , 279 :516 , 2000 |
| Abstract : Gopal_2000_Biochem.Biophys.Res.Commun_279_516 |
| ESTHER : Gopal_2000_Biochem.Biophys.Res.Commun_279_516 |
| PubMedSearch : Gopal_2000_Biochem.Biophys.Res.Commun_279_516 |
| PubMedID: 11118318 |
| Title : Organophosphorus acid anhydride hydrolase activity in human butyrylcholinesterase: synergy results in a somanase - Millard_1998_Biochemistry_37_237 |
| Author(s) : Millard CB , Lockridge O , Broomfield CA |
| Ref : Biochemistry , 37 :237 , 1998 |
| Abstract : Millard_1998_Biochemistry_37_237 |
| ESTHER : Millard_1998_Biochemistry_37_237 |
| PubMedSearch : Millard_1998_Biochemistry_37_237 |
| PubMedID: 9425044 |
| Gene_locus related to this paper: human-BCHE |
| Title : A single amino acid substitution, Gly117His, confers phosphotriesterase (organophosphorus acid anhydride hydrolase) activity on human butyrylcholinesterase - Lockridge_1997_Biochemistry_36_786 |
| Author(s) : Lockridge O , Blong RM , Masson P , Froment MT , Millard CB , Broomfield CA |
| Ref : Biochemistry , 36 :786 , 1997 |
| Abstract : Lockridge_1997_Biochemistry_36_786 |
| ESTHER : Lockridge_1997_Biochemistry_36_786 |
| PubMedSearch : Lockridge_1997_Biochemistry_36_786 |
| PubMedID: 9020776 |
| Title : Design and expression of organophosphorus acid anhydride hydrolase activity in human butyrylcholinesterase - Millard_1995_Biochemistry_34_15925 |
| Author(s) : Millard CB , Lockridge O , Broomfield CA |
| Ref : Biochemistry , 34 :15925 , 1995 |
| Abstract : Millard_1995_Biochemistry_34_15925 |
| ESTHER : Millard_1995_Biochemistry_34_15925 |
| PubMedSearch : Millard_1995_Biochemistry_34_15925 |
| PubMedID: 8519749 |
| Title : The crystal and molecular structure of the Rhizomucor miehei triacylglyceride lipase at 1.9 A resolution - Derewenda_1992_J.Mol.Biol_227_818 |
| Author(s) : Derewenda ZS , Derewenda U , Dodson GG |
| Ref : Journal of Molecular Biology , 227 :818 , 1992 |
| Abstract : Derewenda_1992_J.Mol.Biol_227_818 |
| ESTHER : Derewenda_1992_J.Mol.Biol_227_818 |
| PubMedSearch : Derewenda_1992_J.Mol.Biol_227_818 |
| PubMedID: 1404390 |
| Gene_locus related to this paper: rhimi-lipas |
| Title : Short-term effects of paraoxon and atropine on schedule-controlled behavior in rats - Chambers_1989_Neurotoxicol.Teratol_11_427 |
| Author(s) : Chambers JE , Chambers HW |
| Ref : Neurotoxicology & Teratology , 11 :427 , 1989 |
| Abstract : Chambers_1989_Neurotoxicol.Teratol_11_427 |
| ESTHER : Chambers_1989_Neurotoxicol.Teratol_11_427 |
| PubMedSearch : Chambers_1989_Neurotoxicol.Teratol_11_427 |
| PubMedID: 2593981 |
| Title : Mintacol (diethyl-p-nitrophenylphosphate) (Paraoxon) - |
| Author(s) : Augustinsson KB |
| Ref : Sven Farm Tidskr , 57 :261 , 1953 |
| PubMedID: 13077191 |
| Title : Enzyme studies with diethyl-p-nitrophenylphosphate (mintacol) - |
| Author(s) : Augustinsson KB |
| Ref : Acta Pharmacologica et Toxicologica (Copenh) , 9 :245 , 1953 |
| PubMedID: 13123943 |