Remimazolam
General
Type : Benzodiazepin || Not A\/B H target || Drug || Propionate || Azepine
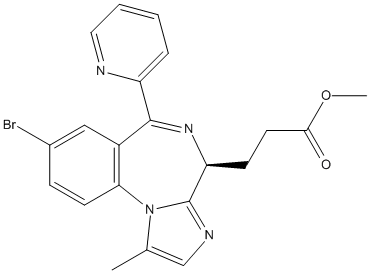
Chemical_Nomenclature : methyl 3-[(4S)-8-bromo-1-methyl-6-pyridin-2-yl-4H-imidazo[1,2-a][1,4]benzodiazepin-4-yl]propanoate
Canonical SMILES : CC1=CN=C2N1C3=C(C=C(C=C3)Br)C(=NC2CCC(=O)OC)C4=CC=CC=N4
InChI : InChI=1S\/C21H19BrN4O2\/c1-13-12-24-21-17(7-9-19(27)28-2)25-20(16-5-3-4-10-23-16)15-11-14(22)6-8-18(15)26(13)21\/h3-6,8,10-12,17H,7,9H2,1-2H3\/t17-\/m0\/s1
InChIKey : CYHWMBVXXDIZNZ-KRWDZBQOSA-N
Other name(s) : ChEMBL4297526, DB12404, CNS 7056, UNII-7V4A8U16MB, CNS-7056, Byfavo
Target
Families : Carb_B_Chordata
References (6)
| Title : The Metabolism of the New Benzodiazepine Remimazolam - Schmalix_2024_Curr.Drug.Metab__ |
| Author(s) : Schmalix W , Petersen KU , Pesic M , Stohr T |
| Ref : Curr Drug Metab , : , 2024 |
| Abstract : Schmalix_2024_Curr.Drug.Metab__ |
| ESTHER : Schmalix_2024_Curr.Drug.Metab__ |
| PubMedSearch : Schmalix_2024_Curr.Drug.Metab__ |
| PubMedID: 38523539 |
| Title : Remimazolam and serious adverse events: A scoping review - Kempenaers_2023_Eur.J.Anaesthesiol__ |
| Author(s) : Kempenaers S , Hansen TG , Van de Velde M |
| Ref : European Journal of Anaesthesiologyiol , : , 2023 |
| Abstract : Kempenaers_2023_Eur.J.Anaesthesiol__ |
| ESTHER : Kempenaers_2023_Eur.J.Anaesthesiol__ |
| PubMedSearch : Kempenaers_2023_Eur.J.Anaesthesiol__ |
| PubMedID: 37727906 |
| Title : Anesthetic Management Using Remimazolam in a Hemodialysis Patient - Nishioka_2023_Anesth.Prog_70_65 |
| Author(s) : Nishioka Y , Miyake S , Hamaoka M , Miyake K , Fujimoto M , Higuchi H , Miyawaki T |
| Ref : Anesth Prog , 70 :65 , 2023 |
| Abstract : Nishioka_2023_Anesth.Prog_70_65 |
| ESTHER : Nishioka_2023_Anesth.Prog_70_65 |
| PubMedSearch : Nishioka_2023_Anesth.Prog_70_65 |
| PubMedID: 37379088 |
| Title : Remimazolam: pharmacological characteristics and clinical applications in anesthesiology - Kim_2022_Anesth.Pain.Med.(Seoul)_17_1 |
| Author(s) : Kim KM |
| Ref : Anesth Pain Med (Seoul) , 17 :1 , 2022 |
| Abstract : Kim_2022_Anesth.Pain.Med.(Seoul)_17_1 |
| ESTHER : Kim_2022_Anesth.Pain.Med.(Seoul)_17_1 |
| PubMedSearch : Kim_2022_Anesth.Pain.Med.(Seoul)_17_1 |
| PubMedID: 35139608 |
| Title : A New Anesthetic, Remimazolam, Is Useful in the Management of Anesthesia in Patients with Liver Cirrhosis - Onoda_2022_Case.Rep.Anesthesiol_2022_9268454 |
| Author(s) : Onoda A , Suzuki Y |
| Ref : Case Rep Anesthesiol , 2022 :9268454 , 2022 |
| Abstract : Onoda_2022_Case.Rep.Anesthesiol_2022_9268454 |
| ESTHER : Onoda_2022_Case.Rep.Anesthesiol_2022_9268454 |
| PubMedSearch : Onoda_2022_Case.Rep.Anesthesiol_2022_9268454 |
| PubMedID: 35578641 |
| Title : Metabolism of remimazolam in primary human hepatocytes during continuous long-term infusion in a 3-D bioreactor system - Freyer_2019_Drug.Des.Devel.Ther_13_1033 |
| Author(s) : Freyer N , Knospel F , Damm G , Greuel S , Schneider C , Seehofer D , Stohr T , Petersen KU , Zeilinger K |
| Ref : Drug Des Devel Ther , 13 :1033 , 2019 |
| Abstract : Freyer_2019_Drug.Des.Devel.Ther_13_1033 |
| ESTHER : Freyer_2019_Drug.Des.Devel.Ther_13_1033 |
| PubMedSearch : Freyer_2019_Drug.Des.Devel.Ther_13_1033 |
| PubMedID: 31037028 |