Tenofovir-alafenamide
General
Type : Drug || Pro-Drug || Aminopurin || Propionate
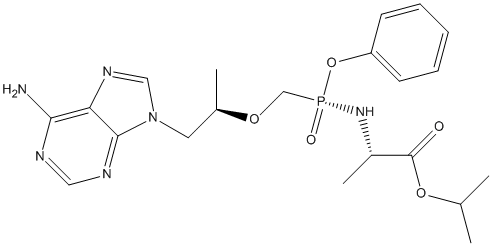
Chemical_Nomenclature : propan-2-yl (2S)-2-[[[(2R)-1-(6-aminopurin-9-yl)propan-2-yl]oxymethyl-phenoxyphosphoryl]amino]propanoate
Canonical SMILES : CC(C)OC(=O)C(C)NP(=O)(COC(C)CN1C=NC2=C(N=CN=C21)N)OC3=CC=CC=C3
InChI : InChI=1S\/C21H29N6O5P\/c1-14(2)31-21(28)16(4)26-33(29,32-17-8-6-5-7-9-17)13-30-15(3)10-27-12-25-18-19(22)23-11-24-20(18)27\/h5-9,11-12,14-16H,10,13H2,1-4H3,(H,26,29)(H2,22,23,24)\/t15-,16+,33+\/m1\/s1
InChIKey : LDEKQSIMHVQZJK-CAQYMETFSA-N
Other name(s) : Tenofovir alafenamide, GS-7340, UNII-J4414G3BUK, J4414G3BUK
Target
Families : Carboxypeptidase_S10, Carb_B_Chordata
References (7)
| Title : Effect of alcohol exposure on the efficacy and safety of tenofovir alafenamide fumarate, a major medicine against human immunodeficiency virus - Liu_2022_Biochem.Pharmacol_204_115224 |
| Author(s) : Liu W , Yu S , Yan B |
| Ref : Biochemical Pharmacology , 204 :115224 , 2022 |
| Abstract : Liu_2022_Biochem.Pharmacol_204_115224 |
| ESTHER : Liu_2022_Biochem.Pharmacol_204_115224 |
| PubMedSearch : Liu_2022_Biochem.Pharmacol_204_115224 |
| PubMedID: 36007574 |
| Title : Contributions of Cathepsin A and Carboxylesterase 1 to the hydrolysis of Tenofovir Alafenamide in the Human Liver, and the Effect of CES1 Genetic Variation on Tenofovir Alafenamide Hydrolysis - Li_2021_Drug.Metab.Dispos__ |
| Author(s) : Li J , Shi J , Xiao J , Tran L , Wang X , Zhu HJ |
| Ref : Drug Metabolism & Disposition: The Biological Fate of Chemicals , : , 2021 |
| Abstract : Li_2021_Drug.Metab.Dispos__ |
| ESTHER : Li_2021_Drug.Metab.Dispos__ |
| PubMedSearch : Li_2021_Drug.Metab.Dispos__ |
| PubMedID: 34933885 |
| Gene_locus related to this paper: human-CES1 , human-CTSA |
| Title : Activation of Tenofovir Alafenamide and Sofosbuvir in the Human Lung and Its Implications in the Development of Nucleoside\/Nucleotide Prodrugs for Treating SARS-CoV-2 Pulmonary Infection - Li_2021_Pharmaceutics_13_ |
| Author(s) : Li J , Liu S , Shi J , Zhu HJ |
| Ref : Pharmaceutics , 13 : , 2021 |
| Abstract : Li_2021_Pharmaceutics_13_ |
| ESTHER : Li_2021_Pharmaceutics_13_ |
| PubMedSearch : Li_2021_Pharmaceutics_13_ |
| PubMedID: 34683949 |
| Gene_locus related to this paper: human-CTSA |
| Title : Intracellular Activation of Tenofovir Alafenamide and the Effect of Viral and Host Protease Inhibitors - Birkus_2015_Antimicrob.Agents.Chemother_60_316 |
| Author(s) : Birkus G , Bam RA , Willkom M , Frey CR , Tsai L , Stray KM , Yant SR , Cihlar T |
| Ref : Antimicrobial Agents & Chemotherapy , 60 :316 , 2015 |
| Abstract : Birkus_2015_Antimicrob.Agents.Chemother_60_316 |
| ESTHER : Birkus_2015_Antimicrob.Agents.Chemother_60_316 |
| PubMedSearch : Birkus_2015_Antimicrob.Agents.Chemother_60_316 |
| PubMedID: 26503655 |
| Gene_locus related to this paper: human-CES1 |
| Title : Implications of Efficient Hepatic Delivery by Tenofovir Alafenamide (GS-7340) for Hepatitis B Virus Therapy - Murakami_2015_Antimicrob.Agents.Chemother_59_3563 |
| Author(s) : Murakami E , Wang T , Park Y , Hao J , Lepist EI , Babusis D , Ray AS |
| Ref : Antimicrobial Agents & Chemotherapy , 59 :3563 , 2015 |
| Abstract : Murakami_2015_Antimicrob.Agents.Chemother_59_3563 |
| ESTHER : Murakami_2015_Antimicrob.Agents.Chemother_59_3563 |
| PubMedSearch : Murakami_2015_Antimicrob.Agents.Chemother_59_3563 |
| PubMedID: 25870059 |
| Gene_locus related to this paper: human-CTSA |
| Title : Metabolism and antiretroviral activity of tenofovir alafenamide in CD4+ T-cells and macrophages from demographically diverse donors - Bam_2014_Antivir.Ther_19_669 |
| Author(s) : Bam RA , Birkus G , Babusis D , Cihlar T , Yant SR |
| Ref : Antivir Ther , 19 :669 , 2014 |
| Abstract : Bam_2014_Antivir.Ther_19_669 |
| ESTHER : Bam_2014_Antivir.Ther_19_669 |
| PubMedSearch : Bam_2014_Antivir.Ther_19_669 |
| PubMedID: 24625459 |
| Gene_locus related to this paper: human-CTSA |
| Title : Activation of 9-[(R)-2-[[(S)-[[(S)-1-(Isopropoxycarbonyl)ethyl]amino] phenoxyphosphinyl]-methoxy]propyl]adenine (GS-7340) and other tenofovir phosphonoamidate prodrugs by human proteases - Birkus_2008_Mol.Pharmacol_74_92 |
| Author(s) : Birkus G , Kutty N , He GX , Mulato A , Lee W , McDermott M , Cihlar T |
| Ref : Molecular Pharmacology , 74 :92 , 2008 |
| Abstract : Birkus_2008_Mol.Pharmacol_74_92 |
| ESTHER : Birkus_2008_Mol.Pharmacol_74_92 |
| PubMedSearch : Birkus_2008_Mol.Pharmacol_74_92 |
| PubMedID: 18430788 |
| Gene_locus related to this paper: human-CTSA |