o-NTFNAC
General
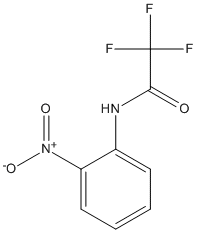
Type : Trifluoro || Acetanilide || 2-nitrophenyl
Chemical_Nomenclature : 2,2,2-Trifluoro-N-(2-nitrophenyl)acetamide
Canonical SMILES : C1=CC=C(C(=C1)NC(=O)C(F)(F)F)[N+](=O)[O-]
InChI : InChI=1S\/C8H5F3N2O3\/c9-8(10,11)7(14)12-5-3-1-2-4-6(5)13(15)16\/h1-4H,(H,12,14)
InChIKey : RDHSJZAEIBUROF-UHFFFAOYSA-N
Other name(s) : AC1MWVTC, O-nitrotrifluoroacetanilide, SCHEMBL5103222, N-(Trifluoroacetyl)-2-nitroaniline, ZINC5514958, N-(2-nitrophenyl)trifluoroacetamide
MW : 234.13
Formula : C8H5F3N2O3
CAS_number :
PubChem :
UniChem :
Iuphar :
References (5)
| Title : Understanding the molecular mechanism of aryl acylamidase activity of acetylcholinesterase - An in silico study - Chinnadurai_2015_Arch.Biochem.Biophys_580_1 |
| Author(s) : Chinnadurai RK , Saravanaraman P , Boopathy R |
| Ref : Archives of Biochemistry & Biophysics , 580 :1 , 2015 |
| Abstract : Chinnadurai_2015_Arch.Biochem.Biophys_580_1 |
| ESTHER : Chinnadurai_2015_Arch.Biochem.Biophys_580_1 |
| PubMedSearch : Chinnadurai_2015_Arch.Biochem.Biophys_580_1 |
| PubMedID: 26072115 |
| Title : Hydrolysis of acetylthiocoline, o-nitroacetanilide and o-nitrotrifluoroacetanilide by fetal bovine serum acetylcholinesterase - Montenegro_2009_FEBS.J_276_2074 |
| Author(s) : Montenegro MF , Moral-Naranjo MT , Munoz-Delgado E , Campoy FJ , Vidal CJ |
| Ref : Febs J , 276 :2074 , 2009 |
| Abstract : Montenegro_2009_FEBS.J_276_2074 |
| ESTHER : Montenegro_2009_FEBS.J_276_2074 |
| PubMedSearch : Montenegro_2009_FEBS.J_276_2074 |
| PubMedID: 19292875 |
| Title : Aryl acylamidase activity of human serum albumin with o-nitrotrifluoroacetanilide as the substrate - Masson_2007_J.Enzyme.Inhib.Med.Chem_22_463 |
| Author(s) : Masson P , Froment MT , Darvesh S , Schopfer LM , Lockridge O |
| Ref : J Enzyme Inhib Med Chem , 22 :463 , 2007 |
| Abstract : Masson_2007_J.Enzyme.Inhib.Med.Chem_22_463 |
| ESTHER : Masson_2007_J.Enzyme.Inhib.Med.Chem_22_463 |
| PubMedSearch : Masson_2007_J.Enzyme.Inhib.Med.Chem_22_463 |
| PubMedID: 17847714 |
| Title : Kinetic analysis of butyrylcholinesterase-catalyzed hydrolysis of acetanilides - Masson_2007_Biochim.Biophys.Acta_1774_1139 |
| Author(s) : Masson P , Froment MT , Gillon E , Nachon F , Darvesh S , Schopfer LM |
| Ref : Biochimica & Biophysica Acta , 1774 :1139 , 2007 |
| Abstract : Masson_2007_Biochim.Biophys.Acta_1774_1139 |
| ESTHER : Masson_2007_Biochim.Biophys.Acta_1774_1139 |
| PubMedSearch : Masson_2007_Biochim.Biophys.Acta_1774_1139 |
| PubMedID: 17690023 |
| Title : On the active site for hydrolysis of aryl amides and choline esters by human cholinesterases - Darvesh_2006_Bioorg.Med.Chem_14_4586 |
| Author(s) : Darvesh S , McDonald RS , Darvesh KV , Mataija D , Mothana S , Cook H , Carneiro KM , Richard N , Walsh R , Martin E |
| Ref : Bioorganic & Medicinal Chemistry , 14 :4586 , 2006 |
| Abstract : Darvesh_2006_Bioorg.Med.Chem_14_4586 |
| ESTHER : Darvesh_2006_Bioorg.Med.Chem_14_4586 |
| PubMedSearch : Darvesh_2006_Bioorg.Med.Chem_14_4586 |
| PubMedID: 16504521 |