DTNB
This chemiluminescent probe is not substrate but react with a product of reaction of a substrate hydrolyzed by an enzyme: this compound reacts with thiocholine after hydrolysis of acetylthiocholine by cholinesterases. A standard reagent for the determination of reactive sulfhydryl groups by absorbance measurements. It is used primarily for the determination of sulfhydryl and disulfide groups in proteins. The color produced is due to the formation of a thio anion, 3-carboxyl-4-nitrothiophenolate
General
Type : Chemiluminescent Probe, Sulfur Compound, Thiocholine reactant
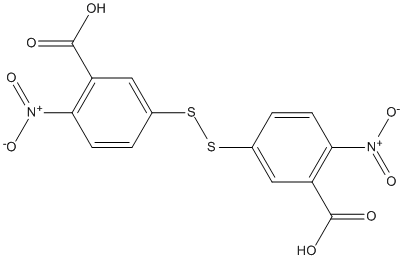
Chemical_Nomenclature : 5-[(3-carboxy-4-nitrophenyl)disulfanyl]-2-nitrobenzoic acid
Canonical SMILES : C1=CC(=C(C=C1SSC2=CC(=C(C=C2)[N+](=O)[O-])C(=O)O)C(=O)O)[N+](=O)[O-]
InChI : InChI=1S\/C14H8N2O8S2\/c17-13(18)9-5-7(1-3-11(9)15(21)22)25-26-8-2-4-12(16(23)24)10(6-8)14(19)20\/h1-6H,(H,17,18)(H,19,20)
InChIKey : KIUMMUBSPKGMOY-UHFFFAOYSA-N
Other name(s) : 5,5'-Dithiobis(2-nitrobenzoic acid) || Ellman's Reagent || 3-Carboxy-4-nitrophenyl disulfide || CHEMBL395814 || CHEBI:86228 || SCHEMBL26775 || ZINC2041301
MW : 396.4
Formula : C14H8N2O8S2
CAS_number : 69-78-3
PubChem : 6254
UniChem : KIUMMUBSPKGMOY-UHFFFAOYSA-N
Target
Structures : No structure
Families : No family
References (15)
| Title : Sulfhydryl reactivity in the central nervous system: effects of electro-shock - |
| Author(s) : Ellman GL , Sullivan CV |
| Ref : Experimental Neurology , 13 :191 , 1965 |
| PubMedID: 5838162 |
| Title : Sulfhydryl reactivity in the central nervous system: effects of electro-shock - |
| Author(s) : Ellman GL , Sullivan CV |
| Ref : Experimental Neurology , 13 :191 , 1965 |
| PubMedID: 5838162 |
| Title : Sulfhydryl reactivity in the central nervous system: effects of electro-shock - |
| Author(s) : Ellman GL , Sullivan CV |
| Ref : Experimental Neurology , 13 :191 , 1965 |
| PubMedID: 5838162 |
| Title : A new and rapid colorimetric determination of acetylcholinesterase activity - Ellman_1961_Biochem.Pharmacol_7_88 |
| Author(s) : Ellman GL , Courtney KD , Andres V , Featherstone RM , Andres V, Jr. |
| Ref : Biochemical Pharmacology , 7 :88 , 1961 |
| Abstract : |
| PubMedSearch : Ellman_1961_Biochem.Pharmacol_7_88 |
| PubMedID: 13726518 |
| Title : A new and rapid colorimetric determination of acetylcholinesterase activity - Ellman_1961_Biochem.Pharmacol_7_88 |
| Author(s) : Ellman GL , Courtney KD , Andres V , Featherstone RM , Andres V, Jr. |
| Ref : Biochemical Pharmacology , 7 :88 , 1961 |
| Abstract : |
| PubMedSearch : Ellman_1961_Biochem.Pharmacol_7_88 |
| PubMedID: 13726518 |
| Title : A new and rapid colorimetric determination of acetylcholinesterase activity - Ellman_1961_Biochem.Pharmacol_7_88 |
| Author(s) : Ellman GL , Courtney KD , Andres V , Featherstone RM , Andres V, Jr. |
| Ref : Biochemical Pharmacology , 7 :88 , 1961 |
| Abstract : |
| PubMedSearch : Ellman_1961_Biochem.Pharmacol_7_88 |
| PubMedID: 13726518 |