Me-GlcA-X
When hydrolysed by Glucuronoyl_esterase (removal of the methyl group) gives a chromogenic substrate for beta-Glucuronidase. The product 5-bromo-4-chloro-3-indolyl react rapidly with oxygen to yield insoluble blue indigo
General
Type : Glucuronoyl, Carbohydrate, Glycoside, Organochlorine
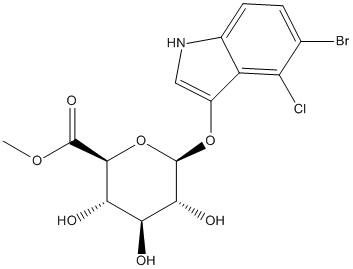
Chemical_Nomenclature : Methyl (5-bromo-4-chloro-3-indolyl beta-D-glucopyranosid)uronate
Canonical SMILES : C1(C(C(C(C(O1)OC2=C[N]C3=CC=C(C(=C23)Cl)Br)O)O)O)C(=O)OC
InChI : InChI=1S\/C15H15BrClNO7\/c1-23-14(22)13-11(20)10(19)12(21)15(25-13)24-7-4-18-6-3-2-5(16)9(17)8(6)7\/h2-4,10-13,15,18-21H,1H3
InChIKey : HKXDXRGOJKQXDY-UHFFFAOYSA-N
Other name(s) : methyl(5-Bromo-4-chloro-3-indolyl beta-d-glucuronide)uronate
Target
Structures : No structure
Families : Glucuronoyl_esterase
References (6)
| Title : beta-Glucuronidase-coupled assays of glucuronoyl esterases - Franova_2016_Anal.Biochem_510_114 |
| Author(s) : Franova L , Puchart V , Biely P |
| Ref : Analytical Biochemistry , 510 :114 , 2016 |
| Abstract : |
| PubMedSearch : Franova_2016_Anal.Biochem_510_114 |
| PubMedID: 27452816 |
| Title : beta-Glucuronidase-coupled assays of glucuronoyl esterases - Franova_2016_Anal.Biochem_510_114 |
| Author(s) : Franova L , Puchart V , Biely P |
| Ref : Analytical Biochemistry , 510 :114 , 2016 |
| Abstract : |
| PubMedSearch : Franova_2016_Anal.Biochem_510_114 |
| PubMedID: 27452816 |
| Title : beta-Glucuronidase-coupled assays of glucuronoyl esterases - Franova_2016_Anal.Biochem_510_114 |
| Author(s) : Franova L , Puchart V , Biely P |
| Ref : Analytical Biochemistry , 510 :114 , 2016 |
| Abstract : |
| PubMedSearch : Franova_2016_Anal.Biochem_510_114 |
| PubMedID: 27452816 |
| Title : Indigogenic substrates for detection and localization of enzymes - Kiernan_2007_Biotech.Histochem_82_73 |
| Author(s) : Kiernan JA |
| Ref : Biotech Histochem , 82 :73 , 2007 |
| Abstract : |
| PubMedSearch : Kiernan_2007_Biotech.Histochem_82_73 |
| PubMedID: 17577701 |
| Title : Indigogenic substrates for detection and localization of enzymes - Kiernan_2007_Biotech.Histochem_82_73 |
| Author(s) : Kiernan JA |
| Ref : Biotech Histochem , 82 :73 , 2007 |
| Abstract : |
| PubMedSearch : Kiernan_2007_Biotech.Histochem_82_73 |
| PubMedID: 17577701 |
| Title : Indigogenic substrates for detection and localization of enzymes - Kiernan_2007_Biotech.Histochem_82_73 |
| Author(s) : Kiernan JA |
| Ref : Biotech Histochem , 82 :73 , 2007 |
| Abstract : |
| PubMedSearch : Kiernan_2007_Biotech.Histochem_82_73 |
| PubMedID: 17577701 |