Antibact-product-Oxadiazolone-cp3
General
Type : Oxadiazol,Carbamate
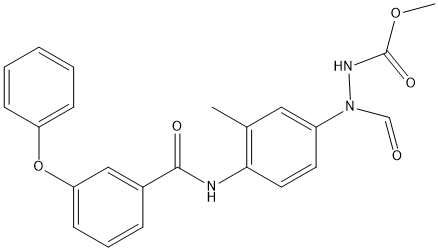
Chemical_Nomenclature : methyl ~{N}-[methanoyl-[3-methyl-4-[(3-phenoxyphenyl)carbonylamino]phenyl]amino]carbamate
Canonical SMILES : Cc1cc(ccc1NC(=O)c2cccc(c2)Oc3ccccc3)N(C=O)NC(=O)OC
InChI : InChI=1S\/C23H21N3O5\/c1-16-13-18(26(15-27)25-23(29)30-2)11-12-21(16)24-22(28)17-7-6-10-20(14-17)31-19-8-4-3-5-9-19\/h3-15H,1-2H3,(H,24,28)(H,25,29)
InChIKey : IFPAECUTANEMNN-UHFFFAOYSA-N
Other name(s) : YKF
MW : 419.43
Formula : C23H21N3O5
CAS_number :
PubChem :
UniChem : IFPAECUTANEMNN-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
Families : Antibact-product-Oxadiazolone-cp3 ligand of proteins in family: 6_AlphaBeta_hydrolase
Stucture : 8G49 FphE, Staphylococcus aureus fluorophosphonate-binding serine hydrolases E, Oxadiazolone compound 3 bound
Protein : staau-SA2367
References (1)
| Title : Chemical Proteomics Reveals Antibiotic Targets of Oxadiazolones in MRSA - Bakker_2023_J.Am.Chem.Soc_145_1136 |
| Author(s) : Bakker AT , Kotsogianni I , Mirenda L , Straub VM , Avalos M , van den Berg R , Florea BI , van Wezel GP , Janssen APA , Martin NI , van der Stelt M |
| Ref : Journal of the American Chemical Society , 145 :1136 , 2023 |
| Abstract : Bakker_2023_J.Am.Chem.Soc_145_1136 |
| ESTHER : Bakker_2023_J.Am.Chem.Soc_145_1136 |
| PubMedSearch : Bakker_2023_J.Am.Chem.Soc_145_1136 |
| PubMedID: 36584241 |
| Gene_locus related to this paper: staa3-q2fkj0 , staau-MW0741 , staau-MW2456 , staau-SA1143 , staau-SA2367 , staau-SAV0321 , staau-SAV0655 |