p-phenylazophenyltrimethylammonium
General
Type : Photoswitch,Trimethylammonium,Not A\/B H target,Nicotinic agonist
Chemical_Nomenclature : trimethyl-(4-phenyldiazenylphenyl)azanium
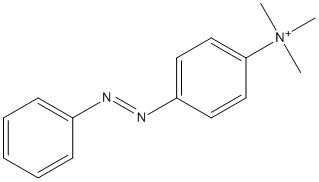
Canonical SMILES : C[N+](C)(C)C1=CC=C(C=C1)N=NC2=CC=CC=C2
InChI : InChI=1S\/C15H18N3\/c1-18(2,3)15-11-9-14(10-12-15)17-16-13-7-5-4-6-8-13\/h4-12H,1-3H3\/q+1
InChIKey : CSBCSRPWHQQFCU-UHFFFAOYSA-N
Other name(s) : p-Phenylazophenyltrimethylammonium,4-Phenylazophenyltrimethylammonium,p-Phenylazophenyltrimethylammonium chloride
MW : 240.32
Formula : C15H18N3+
CAS_number : 3867-72-9
PubChem : 151151
UniChem : CSBCSRPWHQQFCU-UHFFFAOYSA-N
Target
References (3)
| Title : Optical control of acetylcholinesterase with a tacrine switch - Broichhagen_2014_Angew.Chem.Int.Ed.Engl_53_7657 |
| Author(s) : Broichhagen J , Jurastow I , Iwan K , Kummer W , Trauner D |
| Ref : Angew Chem Int Ed Engl , 53 :7657 , 2014 |
| Abstract : Broichhagen_2014_Angew.Chem.Int.Ed.Engl_53_7657 |
| ESTHER : Broichhagen_2014_Angew.Chem.Int.Ed.Engl_53_7657 |
| PubMedSearch : Broichhagen_2014_Angew.Chem.Int.Ed.Engl_53_7657 |
| PubMedID: 24895330 |
| Title : Photoregulation of biological activity by photochromic reagents. 3. Photoregulation of bioelectricity by acetylcholine receptor inhibitors - Deal_1969_Proc.Natl.Acad.Sci.U.S.A_64_1230 |
| Author(s) : Deal WJ , Erlanger BF , Nachmansohn D |
| Ref : Proc Natl Acad Sci U S A , 64 :1230 , 1969 |
| Abstract : Deal_1969_Proc.Natl.Acad.Sci.U.S.A_64_1230 |
| ESTHER : Deal_1969_Proc.Natl.Acad.Sci.U.S.A_64_1230 |
| PubMedSearch : Deal_1969_Proc.Natl.Acad.Sci.U.S.A_64_1230 |
| PubMedID: 5271749 |
| Title : Photoregulation of biological activity by photocromic reagents. II. Inhibitors of acetylcholinesterase - Bieth_1969_Proc.Natl.Acad.Sci.U.S.A_64_1103 |
| Author(s) : Bieth J , Vratsanos SM , Wassermann N , Erlanger BF |
| Ref : Proc Natl Acad Sci U S A , 64 :1103 , 1969 |
| Abstract : Bieth_1969_Proc.Natl.Acad.Sci.U.S.A_64_1103 |
| ESTHER : Bieth_1969_Proc.Natl.Acad.Sci.U.S.A_64_1103 |
| PubMedSearch : Bieth_1969_Proc.Natl.Acad.Sci.U.S.A_64_1103 |
| PubMedID: 5264140 |