Benzylpiperidin-methylsulfonyl-indol-acryloyl-phenyl-acetamide-cpd6b
AChE inhibitory effect (IC50 = 49.30nM) slightly less than tacrine and donepezil, while more potent as BuChE inhibitor (IC50 = 80.44 nM)
General
Type : Multitarget,Indole,Carboxamide,Pirimidine,Derivative of Donepezil,Sulfur Compound
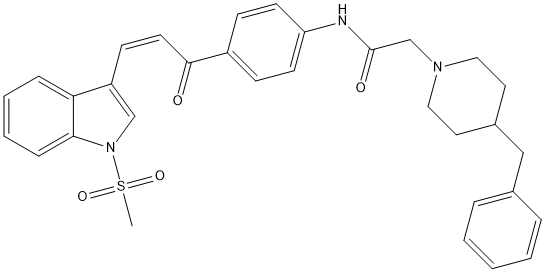
Chemical_Nomenclature : (E)-2(4-Benzylpiperidin-1-yl)-N-{4-[3(1-(methylsulfonyl)-1H-indol-3-yl)acryloyl]phenyl}acetamide
Canonical SMILES : C1=CC=C2C(=C1)C(=C[N]2[S](=O)(=O)C)C=CC(=O)C3=CC=C(C=C3)NC(=O)CN4CCC(CC4)CC5=CC=CC=C5
InChI : InChI=1S\/C32H33N3O4S\/c1-40(38,39)35-22-27(29-9-5-6-10-30(29)35)13-16-31(36)26-11-14-28(15-12-26)33-32(37)23-34-19-17-25(18-20-34)21-24-7-3-2-4-8-24\/h2-16,22,25H,17-21,23H2,1H3,(H,33,37)\/b16-13+
InChIKey : OBYVGGCDILLEFV-DTQAZKPQSA-N
Other name(s) : Compound 6b
MW : 555.69
Formula : C32H33N3O4S
CAS_number :
PubChem :
UniChem : OBYVGGCDILLEFV-DTQAZKPQSA-N
IUPHAR :
Wikipedia :
Target
Families : Benzylpiperidin-methylsulfonyl-indol-acryloyl-phenyl-acetamide-cpd6b ligand of proteins in family: BCHE || ACHE
Stucture :
Protein : human-BCHE || human-ACHE
References (1)
| Title : Design and synthesis of new indole drug candidates to treat Alzheimer's disease and targeting neuro-inflammation using a multi-target-directed ligand (MTDL) strategy - Lamie_2022_J.Enzyme.Inhib.Med.Chem_37_2660 |
| Author(s) : Lamie PF , Abdel-Fattah MM , Philoppes JN |
| Ref : J Enzyme Inhib Med Chem , 37 :2660 , 2022 |
| Abstract : Lamie_2022_J.Enzyme.Inhib.Med.Chem_37_2660 |
| ESTHER : Lamie_2022_J.Enzyme.Inhib.Med.Chem_37_2660 |
| PubMedSearch : Lamie_2022_J.Enzyme.Inhib.Med.Chem_37_2660 |
| PubMedID: 36146947 |