PhX
Similar to VX but much less toxic
General
Type : Organophosphate,Nerve Agent V-series,Sulfur Compound
Chemical_Nomenclature : N-[2-(ethoxy-oxido-phenylphosphaniumyl)sulfanylethyl]-N-propan-2-ylpropan-2-amine
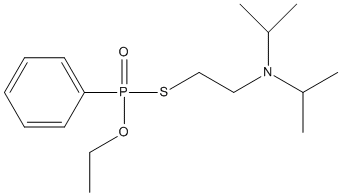
Canonical SMILES : CCO[P+](C1=CC=CC=C1)([O-])SCCN(C(C)C)C(C)C
InChI : InChI=1S\/C16H28NO2PS\/c1-6-19-20(18,16-10-8-7-9-11-16)21-13-12-17(14(2)3)15(4)5\/h7-11,14-15H,6,12-13H2,1-5H3
InChIKey : WYYFXVICAAXHBI-UHFFFAOYSA-N
Other name(s) : S-[2-(diethylamino)ethyl] O-2-phenylpropyl ester,Phenylthiophosphonic acid O-ethyl S-[2-(diisopropylamino)ethyl] ester
MW : 329.44
Formula : C16H28NO2PS
CAS_number :
PubChem : 101019430
UniChem : WYYFXVICAAXHBI-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
References (2)
| Title : Easy access to phosphonothioates - Renard_2002_Chemistry_8_2910 |
| Author(s) : Renard PY , Schwebel H , Vayron P , Josien L , Valleix A , Mioskowski C |
| Ref : Chemistry , 8 :2910 , 2002 |
| Abstract : Renard_2002_Chemistry_8_2910 |
| ESTHER : Renard_2002_Chemistry_8_2910 |
| PubMedSearch : Renard_2002_Chemistry_8_2910 |
| PubMedID: 12489219 |
| Title : Toward antibody-catalyzed hydrolysis of organophosphorus poisons - Vayron_2000_Proc.Natl.Acad.Sci.U.S.A_97_7058 |
| Author(s) : Vayron P , Renard PY , Taran F , Creminon C , Frobert Y , Grassi J , Mioskowski C |
| Ref : Proc Natl Acad Sci U S A , 97 :7058 , 2000 |
| Abstract : Vayron_2000_Proc.Natl.Acad.Sci.U.S.A_97_7058 |
| ESTHER : Vayron_2000_Proc.Natl.Acad.Sci.U.S.A_97_7058 |
| PubMedSearch : Vayron_2000_Proc.Natl.Acad.Sci.U.S.A_97_7058 |
| PubMedID: 10860971 |