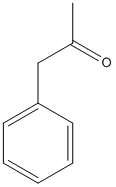
Phenylacetone
For AChE Phenylacetone is a competitive inhibitor of the enzyme but not a transition state analog. Binding constant similar to the substrate phenyl acetate
General
Type : Ketone
Chemical_Nomenclature : 1-phenylpropan-2-one
Canonical SMILES : CC(=O)CC1=CC=CC=C1
InChI : InChI=1S\/C9H10O\/c1-8(10)7-9-5-3-2-4-6-9\/h2-6H,7H2,1H3
InChIKey : QCCDLTOVEPVEJK-UHFFFAOYSA-N
Other name(s) : 1-phenylpropan-2-one,1-Phenyl-2-propanone,Benzyl methyl ketone,Benzyl-methyl-ketone,BMK
MW : 134.17
Formula : C9H10O
CAS_number : 103-79-7
PubChem : 7678
UniChem : QCCDLTOVEPVEJK-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
Families : Phenylacetone ligand of proteins in family: Monoglyceridelipase_lysophospholip || ACHE
Stucture : 6KHL Cutibacterium acnes (Propionibacterium acnes) lipase blocked state stucture inhibited by BMK Benzyl methyl ketone Phenylacetone
Protein : cutac-e6dae5 || eleel-ACHE
References (2)
| Title : The grease trap: Uncovering the mechanism of the hydrophobic lid in Cutibacterium acnes lipase - Kim_2020_J.Lipid.Res__ |
| Author(s) : Kim HJ , Lee BJ , Kwon AR |
| Ref : J Lipid Res , : , 2020 |
| Abstract : Kim_2020_J.Lipid.Res__ |
| ESTHER : Kim_2020_J.Lipid.Res__ |
| PubMedSearch : Kim_2020_J.Lipid.Res__ |
| PubMedID: 32165394 |
| Gene_locus related to this paper: cutac-e6dae5 |
| Title : The mode of binding of potential transition-state analogs to acetylcholinesterase - Dafforn_1977_Biochim.Biophys.Acta_484_375 |
| Author(s) : Dafforn A , Anderson M , Ash D , Campagna J , Daniel E , Horwood R , Kerr P , Rych G , Zappitelli F |
| Ref : Biochimica & Biophysica Acta , 484 :375 , 1977 |
| Abstract : Dafforn_1977_Biochim.Biophys.Acta_484_375 |
| ESTHER : Dafforn_1977_Biochim.Biophys.Acta_484_375 |
| PubMedSearch : Dafforn_1977_Biochim.Biophys.Acta_484_375 |
| PubMedID: 20963 |