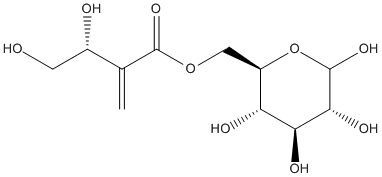
6-Tuliposide-B
tulip lactone-forming carboxylesterases, specifically catalyzing intramolecular transesterification, and convert 6-tuliposide B to D-glucose and tulipalin B
General
Type : Natural, Carbohydrate
Chemical_Nomenclature : [(2R,3S,4S,5R)-3,4,5,6-tetrahydroxyoxan-2-yl]methyl (3S)-3,4-dihydroxy-2-methylidenebutanoate
Canonical SMILES : C=C(C(CO)O)C(=O)OCC1C(C(C(C(O1)O)O)O)O
InChI : InChI=1S\/C11H18O9\/c1-4(5(13)2-12)10(17)19-3-6-7(14)8(15)9(16)11(18)20-6\/h5-9,11-16,18H,1-3H2\/t5-,6-,7-,8+,9-,11?\/m1\/s1
InChIKey : FMHJNIRDGYFPEC-CHICSPHLSA-N
Other name(s) : 6-tuliposide B || 6-O-[(3S)-3,4-Dihydroxy-2-methylenebutanoyl]-D-glucose || CHEBI:87124 || C21186
MW : 294.25
Formula : C11H18O9
CAS_number :
PubChem : 25034024
UniChem : FMHJNIRDGYFPEC-CHICSPHLSA-N
Target
Structures : No structure
Families : Plant_carboxylesterase
References (6)
| Title : Molecular identification of tuliposide B-converting enzyme: a lactone-forming carboxylesterase from the pollen of tulip - Nomura_2015_Plant.J_83_252 |
| Author(s) : Nomura T , Murase T , Ogita S , Kato Y |
| Ref : Plant J , 83 :252 , 2015 |
| Abstract : |
| PubMedSearch : Nomura_2015_Plant.J_83_252 |
| PubMedID: 25997073 |
| Gene_locus related to this paper: tulge-a0a0h5bmx5 |
| Title : Molecular identification of tuliposide B-converting enzyme: a lactone-forming carboxylesterase from the pollen of tulip - Nomura_2015_Plant.J_83_252 |
| Author(s) : Nomura T , Murase T , Ogita S , Kato Y |
| Ref : Plant J , 83 :252 , 2015 |
| Abstract : |
| PubMedSearch : Nomura_2015_Plant.J_83_252 |
| PubMedID: 25997073 |
| Gene_locus related to this paper: tulge-a0a0h5bmx5 |
| Title : Molecular identification of tuliposide B-converting enzyme: a lactone-forming carboxylesterase from the pollen of tulip - Nomura_2015_Plant.J_83_252 |
| Author(s) : Nomura T , Murase T , Ogita S , Kato Y |
| Ref : Plant J , 83 :252 , 2015 |
| Abstract : |
| PubMedSearch : Nomura_2015_Plant.J_83_252 |
| PubMedID: 25997073 |
| Gene_locus related to this paper: tulge-a0a0h5bmx5 |
| Title : The antibacterial properties of 6-tuliposide B. Synthesis of 6-tuliposide B analogues and structure-activity relationship - Shigetomi_2010_Phytochemistry_71_312 |
| Author(s) : Shigetomi K , Shoji K , Mitsuhashi S , Ubukata M |
| Ref : Phytochemistry , 71 :312 , 2010 |
| Abstract : |
| PubMedSearch : Shigetomi_2010_Phytochemistry_71_312 |
| PubMedID: 19939419 |
| Title : The antibacterial properties of 6-tuliposide B. Synthesis of 6-tuliposide B analogues and structure-activity relationship - Shigetomi_2010_Phytochemistry_71_312 |
| Author(s) : Shigetomi K , Shoji K , Mitsuhashi S , Ubukata M |
| Ref : Phytochemistry , 71 :312 , 2010 |
| Abstract : |
| PubMedSearch : Shigetomi_2010_Phytochemistry_71_312 |
| PubMedID: 19939419 |
| Title : The antibacterial properties of 6-tuliposide B. Synthesis of 6-tuliposide B analogues and structure-activity relationship - Shigetomi_2010_Phytochemistry_71_312 |
| Author(s) : Shigetomi K , Shoji K , Mitsuhashi S , Ubukata M |
| Ref : Phytochemistry , 71 :312 , 2010 |
| Abstract : |
| PubMedSearch : Shigetomi_2010_Phytochemistry_71_312 |
| PubMedID: 19939419 |