Chlorimuron
Product of hydrolysis of Chlorimuron-ethyl. An acetolactate synthase inhibitor, it is used (generally as the corresponding ethyl ester) as a herbicide. Chlorimuron-ethyl is an ethyl ester resulting from the formal condensation of the carboxy group of chlorimuron with ethanol. A proherbicide for chloimuron, it is used as herbicide for the control of broad-leaved weeds in peanuts, soya beans, and other crops. An EC 2.2.1.6 (acetolactate synthase) inhibitor. It is a sulfamoylbenzoate, a N-sulfonylurea, an aromatic ether, an ethyl ester, an organochlorine pesticide and a member of pyrimidines. Inhibition of Candida albicans acetohydroxyacid synthase explains fungicide action.
General
Type : Herbicide, Sulfonamide, Benzenesulfonamide, Sulfur Compound, Carboxamide, Urea derivative, Pyrimidine, Benzoate, Organochlorine
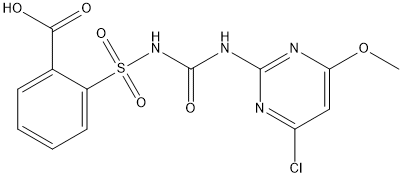
Chemical_Nomenclature : 2-[(4-chloro-6-methoxypyrimidin-2-yl)carbamoylsulfamoyl]benzoic acid
Canonical SMILES : COC1=CC(=NC(=N1)NC(=O)NS(=O)(=O)C2=CC=CC=C2C(=O)O)Cl
InChI : InChI=1S\/C13H11ClN4O6S\/c1-24-10-6-9(14)15-12(16-10)17-13(21)18-25(22,23)8-5-3-2-4-7(8)11(19)20\/h2-6H,1H3,(H,19,20)(H2,15,16,17,18,21)
InChIKey : RIUXZHMCCFLRBI-UHFFFAOYSA-N
Other name(s) : 2-(N-((4-Chloro-6-methoxypyrimidin-2-yl)carbamoyl)sulfamoyl)benzoic acid || IJC
MW : 386.77
Formula : C13H11ClN4O6S
CAS_number : 99283-00-8
PubChem : 91762
UniChem : RIUXZHMCCFLRBI-UHFFFAOYSA-N
Target
Structures : 8GOL
Families : Bacterial_esterase
References (3)
| Title : Crystal structures of herbicide-detoxifying esterase reveal a lid loop affecting substrate binding and activity - Liu_2023_Nat.Commun_14_4343 |
| Author(s) : Liu B , Wang W , Qiu J , Huang X , Qiu S , Bao Y , Xu S , Ruan L , Ran T , He J |
| Ref : Nat Commun , 14 :4343 , 2023 |
| Abstract : |
| PubMedSearch : Liu_2023_Nat.Commun_14_4343 |
| PubMedID: 37468532 |
| Gene_locus related to this paper: 9rhiz-g9i933 |
| Title : Crystal structures of herbicide-detoxifying esterase reveal a lid loop affecting substrate binding and activity - Liu_2023_Nat.Commun_14_4343 |
| Author(s) : Liu B , Wang W , Qiu J , Huang X , Qiu S , Bao Y , Xu S , Ruan L , Ran T , He J |
| Ref : Nat Commun , 14 :4343 , 2023 |
| Abstract : |
| PubMedSearch : Liu_2023_Nat.Commun_14_4343 |
| PubMedID: 37468532 |
| Gene_locus related to this paper: 9rhiz-g9i933 |
| Title : Crystal structures of herbicide-detoxifying esterase reveal a lid loop affecting substrate binding and activity - Liu_2023_Nat.Commun_14_4343 |
| Author(s) : Liu B , Wang W , Qiu J , Huang X , Qiu S , Bao Y , Xu S , Ruan L , Ran T , He J |
| Ref : Nat Commun , 14 :4343 , 2023 |
| Abstract : |
| PubMedSearch : Liu_2023_Nat.Commun_14_4343 |
| PubMedID: 37468532 |
| Gene_locus related to this paper: 9rhiz-g9i933 |