Homosalate
CES2 is the primary isoform responsible for the hydrolysis of all four sunscreen UV filters and is exclusively responsible for the biotransformation of homosalate
General
Type : Sun screen additive, Salicylic, Aryl ester
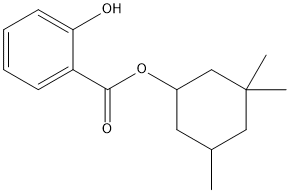
Chemical_Nomenclature : (3,3,5-trimethylcyclohexyl) 2-hydroxybenzoate
Canonical SMILES : CC1CC(CC(C1)(C)C)OC(=O)C2=CC=CC=C2O
InChI : InChI=1S\/C16H22O3\/c1-11-8-12(10-16(2,3)9-11)19-15(18)13-6-4-5-7-14(13)17\/h4-7,11-12,17H,8-10H2,1-3H3
InChIKey : WSSJONWNBBTCMG-UHFFFAOYSA-N
Other name(s) : Homomenthyl salicylate || Heliophan || 3,3,5-Trimethylcyclohexyl 2-hydroxybenzoate\; Homosalato || Homosalatum || (3,3,5-trimethylcyclohexyl) 2-hydroxybenzoate || CHEMBL1377575 || SCHEMBL29356436 || CHEBI:91642 || DB11064
MW : 262.34
Formula : C16H22O3
CAS_number : 118-56-9
PubChem : 8362
UniChem : WSSJONWNBBTCMG-UHFFFAOYSA-N
Target
Structures : No structure
Families : Carb_B_Chordata
References (6)
| Title : Assessing the hepatic metabolism of six sunscreen ultraviolet filters: In vitro-in vivo extrapolation approach using human liver microsomes and hepatic parameter predictions for exposure risk assessment - Baviera_2025_Biochem.Pharmacol__117564 |
| Author(s) : Baviera GS , Poli VR , Oka-Duarte L , Santos Barbetta MF , Moraes de Oliveira AR |
| Ref : Biochemical Pharmacology , :117564 , 2025 |
| Abstract : |
| PubMedSearch : Baviera_2025_Biochem.Pharmacol__117564 |
| PubMedID: 41319926 |
| Title : Assessing the hepatic metabolism of six sunscreen ultraviolet filters: In vitro-in vivo extrapolation approach using human liver microsomes and hepatic parameter predictions for exposure risk assessment - Baviera_2025_Biochem.Pharmacol__117564 |
| Author(s) : Baviera GS , Poli VR , Oka-Duarte L , Santos Barbetta MF , Moraes de Oliveira AR |
| Ref : Biochemical Pharmacology , :117564 , 2025 |
| Abstract : |
| PubMedSearch : Baviera_2025_Biochem.Pharmacol__117564 |
| PubMedID: 41319926 |
| Title : Assessing the hepatic metabolism of six sunscreen ultraviolet filters: In vitro-in vivo extrapolation approach using human liver microsomes and hepatic parameter predictions for exposure risk assessment - Baviera_2025_Biochem.Pharmacol__117564 |
| Author(s) : Baviera GS , Poli VR , Oka-Duarte L , Santos Barbetta MF , Moraes de Oliveira AR |
| Ref : Biochemical Pharmacology , :117564 , 2025 |
| Abstract : |
| PubMedSearch : Baviera_2025_Biochem.Pharmacol__117564 |
| PubMedID: 41319926 |
| Title : Toxicokinetics of homosalate in humans after dermal application: applicability of oral-route data for exposure assessment by human biomonitoring - Ebert_2024_Arch.Toxicol_98_1383 |
| Author(s) : Ebert KE , Griem P , Weiss T , Bruning T , Hayen H , Koch HM , Bury D |
| Ref : Archives of Toxicology , 98 :1383 , 2024 |
| Abstract : |
| PubMedSearch : Ebert_2024_Arch.Toxicol_98_1383 |
| PubMedID: 38485782 |
| Title : Toxicokinetics of homosalate in humans after dermal application: applicability of oral-route data for exposure assessment by human biomonitoring - Ebert_2024_Arch.Toxicol_98_1383 |
| Author(s) : Ebert KE , Griem P , Weiss T , Bruning T , Hayen H , Koch HM , Bury D |
| Ref : Archives of Toxicology , 98 :1383 , 2024 |
| Abstract : |
| PubMedSearch : Ebert_2024_Arch.Toxicol_98_1383 |
| PubMedID: 38485782 |
| Title : Toxicokinetics of homosalate in humans after dermal application: applicability of oral-route data for exposure assessment by human biomonitoring - Ebert_2024_Arch.Toxicol_98_1383 |
| Author(s) : Ebert KE , Griem P , Weiss T , Bruning T , Hayen H , Koch HM , Bury D |
| Ref : Archives of Toxicology , 98 :1383 , 2024 |
| Abstract : |
| PubMedSearch : Ebert_2024_Arch.Toxicol_98_1383 |
| PubMedID: 38485782 |