Isosorbide-2-aspirinate-5-mononitrate
General
Type : Isosorbide, Salicylic, Pro-Drug
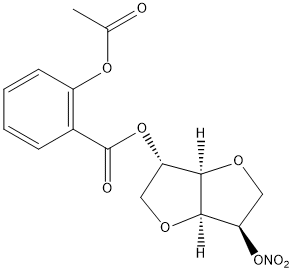
Chemical_Nomenclature : [(3S,3aR,6R,6aS)-6-nitrooxy-2,3,3a,5,6,6a-hexahydrofuro[3,2-b]furan-3-yl] 2-acetyloxybenzoate
Canonical SMILES : CC(=O)OC1=CC=CC=C1C(=O)OC2COC3C2OCC3O[N+](=O)[O-]
InChI : InChI=1S\/C15H15NO9\/c1-8(17)23-10-5-3-2-4-9(10)15(18)24-11-6-21-14-12(25-16(19)20)7-22-13(11)14\/h2-5,11-14H,6-7H2,1H3\/t11-,12+,13+,14+\/m0\/s1
InChIKey : WKOQXDYPKBXKJG-REWJHTLYSA-N
Other name(s) : ISMNA || CHEMBL585137
MW : 353.28
Formula : C15H15NO9
CAS_number :
PubChem : 9863359
UniChem : WKOQXDYPKBXKJG-REWJHTLYSA-N
Target
Structures : No structure
Families : BCHE
References (2)
| Title : Single oral dose study of two isosorbide-based aspirin prodrugs in the dog - Gilmer_2003_J.Pharm.Pharmacol_55_1351 |
| Author(s) : Gilmer JF , Murphy MA , Shannon JA , Breen CG , Ryder SA , Clancy JM |
| Ref : J Pharm Pharmacol , 55 :1351 , 2003 |
| Abstract : |
| PubMedSearch : Gilmer_2003_J.Pharm.Pharmacol_55_1351 |
| PubMedID: 14607016 |
| Title : Synthesis, hydrolysis kinetics and anti-platelet effects of isosorbide mononitrate derivatives of aspirin - Gilmer_2001_Eur.J.Pharm.Sci_14_221 |
| Author(s) : Gilmer JF , Moriarty LM , McCafferty DF , Clancy JM |
| Ref : Eur J Pharm Sci , 14 :221 , 2001 |
| Abstract : |
| PubMedSearch : Gilmer_2001_Eur.J.Pharm.Sci_14_221 |
| PubMedID: 11576827 |