O-UXXR
General
Type : Glucuronoyl || Glycoside
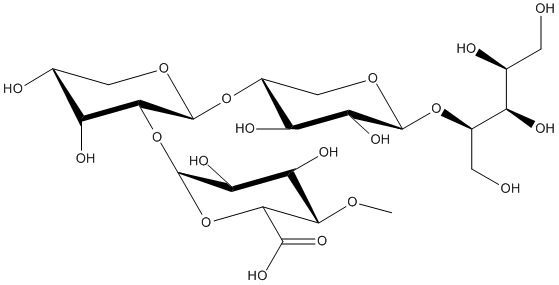
Chemical_Nomenclature : 23-(4-O-Methyl-D-glucuronyl)-alpha-D-xylotriitol
Canonical SMILES : O1[C@H]([C@H](C([C@@H](C1)O)O)O[C@@H]2OC([C@H](C([C@@H]2O)O)OC)C(O)=O)O[C@H]3C([C@@H]([C@@H](OC3)O[C@H](CO)[C@@H]([C@H](CO)O)O)O)O
InChI : InChI=1S\/C22H38O19\/c1-35-16-13(30)15(32)21(41-18(16)19(33)34)40-17-11(28)7(26)4-36-22(17)39-9-5-37-20(14(31)12(9)29)38-8(3-24)10(27)6(25)2-23\/h6-18,20-32H,2-5H2,1H3,(H,33,34)\/t6-,7+,8+,9+,10+,11?,12?,13?,14-,15-,16-,17-,18?,20-,21+,22-\/m0\/s1
InChIKey : WLCUSDSGZFCSGQ-UWDXULLYSA-N
Other name(s) : Aldotetrauronic acid (terminal substitution, borohydride reduced)
Target
Families : Glucuronoyl_esterase
References (1)
| Title : The structural basis of fungal glucuronoyl esterase activity on natural substrates - Ernst_2020_Nat.Commun_11_1026 |
| Author(s) : Ernst HA , Mosbech C , Langkilde AE , Westh P , Meyer AS , Agger JW , Larsen S |
| Ref : Nat Commun , 11 :1026 , 2020 |
| Abstract : Ernst_2020_Nat.Commun_11_1026 |
| ESTHER : Ernst_2020_Nat.Commun_11_1026 |
| PubMedSearch : Ernst_2020_Nat.Commun_11_1026 |
| PubMedID: 32094331 |
| Gene_locus related to this paper: cerui-gce |