Esterastin
General
Type : Oxooxetan,Natural,Lipase inhibitor
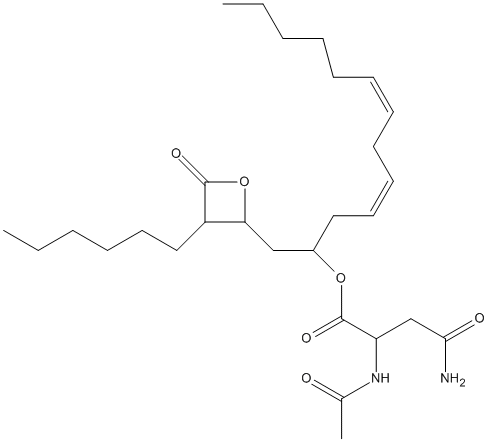
Chemical_Nomenclature : 1-(3-hexyl-4-oxooxetan-2-yl)trideca-4,7-dien-2-yl 2-acetamido-4-amino-4-oxobutanoate
Canonical SMILES : CCCCCCC1C(OC1=O)CC(CC=CCC=CCCCCC)OC(=O)C(CC(=O)N)NC(=O)C
InChI : InChI=1S\/C28H46N2O6\/c1-4-6-8-10-11-12-13-14-15-17-22(35-28(34)24(20-26(29)32)30-21(3)31)19-25-23(27(33)36-25)18-16-9-7-5-2\/h11-12,14-15,22-25H,4-10,13,16-20H2,1-3H3,(H2,29,32)(H,30,31)
InChIKey : JKNGELGDDBUFHG-UHFFFAOYSA-N
Other name(s) : L-Asparagine, N(2)-acetyl-, 1-((3-hexyl-4-oxo-2-oxetanyl)methyl)-3,6-dodecadienyl ester, (2S-(2alpha(1R*,3Z,6Z),3beta))-,L-Asparagine, N(sup 2)-acetyl-, 1-((3-hexyl-4-oxo-2-oxetanyl)methyl)-3,6-dodecadienyl ester, (2S-(2-alpha(1R*,3Z,6Z),3-beta))-,AC1L2OYT
MW : 506.67
Formula : C28H46N2O6
CAS_number : 67655-93-0
PubChem : 125669
UniChem : JKNGELGDDBUFHG-UHFFFAOYSA-N
Target
Families : Esterastin ligand of proteins in family
Acidic_Lipase
Pancreatic_lipase
References (5)
| Title : Relative specificities of inhibition of acid cholesteryl ester hydrolase and neutral cholesteryl ester hydrolase in cultured rabbit aortic smooth muscle cells by esterastin and cholesteryl oleyl ether - Morin_1989_Biochim.Biophys.Acta_1004_139 |
| Author(s) : Morin RJ , Peng SK |
| Ref : Biochimica & Biophysica Acta , 1004 :139 , 1989 |
| Abstract : Morin_1989_Biochim.Biophys.Acta_1004_139 |
| ESTHER : Morin_1989_Biochim.Biophys.Acta_1004_139 |
| PubMedSearch : Morin_1989_Biochim.Biophys.Acta_1004_139 |
| PubMedID: 2742868 |
| Title : Effect of esterastin, an acid lipase inhibitor, on the free and esterified cholesterol contents of cultured aortic smooth muscle cells treated with LDL and cholesterol ester liquid crystals - Ecsedi_1985_Biochem.Int_10_337 |
| Author(s) : Ecsedi GG , Amanuma K , Imanaka T , Aoyagi T , Ohkuma S , Takano T |
| Ref : Biochemistry International , 10 :337 , 1985 |
| Abstract : Ecsedi_1985_Biochem.Int_10_337 |
| ESTHER : Ecsedi_1985_Biochem.Int_10_337 |
| PubMedSearch : Ecsedi_1985_Biochem.Int_10_337 |
| PubMedID: 4015661 |
| Title : Esterastin: a potent inhibitor of lysosomal acid lipase - Imanaka_1983_J.Biochem_94_1017 |
| Author(s) : Imanaka T , Moriyama Y , Ecsedi GG , Aoyagi T , Amanuma-Muto K , Ohkuma S , Takano T |
| Ref : J Biochem , 94 :1017 , 1983 |
| Abstract : Imanaka_1983_J.Biochem_94_1017 |
| ESTHER : Imanaka_1983_J.Biochem_94_1017 |
| PubMedSearch : Imanaka_1983_J.Biochem_94_1017 |
| PubMedID: 6643414 |
| Title : The structure of esterastin, an inhibitor of esterase - |
| Author(s) : Kondo S , Uotani K , Miyamoto M , Hazato T , Naganawa H , Aoyagi T |
| Ref : J Antibiot (Tokyo) , 31 :797 , 1978 |
| PubMedID: 690012 |