Indole-piperidine-amide-cpd23a
Inhibits hAChE and hBACE-1 with IC50 values of 0.32 and 0.39 microM, respectively. ElEelAChE 0.52 microM , eqBCHE 19.16 microM
General
Type : Multitarget,Indole,Carboxamide,Derivative of Donepezil,BACE1-inhibitor,Piperidine
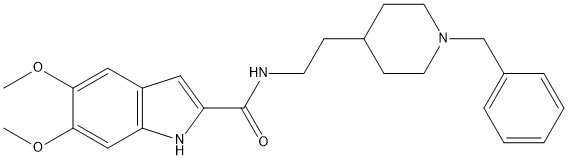
Chemical_Nomenclature : 5,6-dimethoxy-indole N-(2-(1-benzylpiperidine) carboxamide
Canonical SMILES : C12=C([C]=C(C(=[C]1)O[C])O[C])[C]=C([N]2)C([N]CC[C]3CCN(CC3)CC4=[C][C]=[C][C]=[C]4)=O
InChI : InChI=1S\/C25H31N3O3\/c1-30-23-15-20-14-22(27-21(20)16-24(23)31-2)25(29)26-11-8-18-9-12-28(13-10-18)17-19-6-4-3-5-7-19\/h3-7,14-16,18,27H,8-13,17H2,1-2H3,(H,26,29)
InChIKey : MHFAWVHGIRXFHE-UHFFFAOYSA-N
Other name(s) : Compound 23a
MW : 421.53
Formula : C25H31N3O3
CAS_number :
PubChem :
UniChem : MHFAWVHGIRXFHE-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
Families : Indole-piperidine-amide-cpd23a ligand of proteins in family: ACHE
Stucture :
Protein : human-ACHE
References (1)
| Title : Design, synthesis, and pharmacological evaluation of indole-piperidine amides as Blood-brain barrier permeable dual cholinesterase and beta-secretase inhibitors - Banoo_2024_Eur.J.Med.Chem_266_116131 |
| Author(s) : Banoo R , Nuthakki VK , Wadje BN , Sharma A , Bharate SB |
| Ref : Eur Journal of Medicinal Chemistry , 266 :116131 , 2024 |
| Abstract : Banoo_2024_Eur.J.Med.Chem_266_116131 |
| ESTHER : Banoo_2024_Eur.J.Med.Chem_266_116131 |
| PubMedSearch : Banoo_2024_Eur.J.Med.Chem_266_116131 |
| PubMedID: 38215587 |