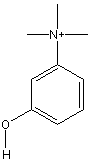
3-Hydroxy-Phenyl-Trimethyl-Ammonium
metabolite of neostigmine, bromide is Ro 2-2561
General
Type : Trimethylammonium
Chemical_Nomenclature : 3-Hydroxy Phenyl Trimethyl Ammonium iodide, Methanesulfonate
Canonical SMILES : C[N+](C)(C)C1=CC(=CC=C1)O.[I-]
InChI : InChI=1S\/C9H13NO.HI\/c1-10(2,3)8-5-4-6-9(11)7-8\;\/h4-7H,1-3H3\;1H
InChIKey : KNPHMCQIKGHPRU-UHFFFAOYSA-N
Other name(s) : TMPH || PTMA || 3-(Trimethylammonio)phenol iodide || m-(Dimethylamino)phenol methiodide || 3-Oxyphenyl trimethylammonium iodide || (m-Hydroxyphenyl)trimethylammonium iodide || 3-Hydroxy-N,N,N-trimethylbenzenaminium iodide || Ro 2-2561
MW : 279.118
Formula : C9H14INO
CAS_number : 2498-27-3
PubChem : 17250
UniChem : KNPHMCQIKGHPRU-UHFFFAOYSA-N
Target
Families : 3-Hydroxy-Phenyl-Trimethyl-Ammonium ligand of proteins in family
ACHE
References (2)
| Title : Inhibition of acetylcholinesterase by hemicholiniums, conformationally constrained choline analogues. Evaluation of aryl and alkyl substituents. Comparisons with choline and (3- hydroxyphenyl)trimethylammonium - Lee_1992_Chem.Res.Toxicol_5_411 |
| Author(s) : Lee BH , Stelly TC , Colucci WJ , Garcia JG , Gandour RD , Quinn DM |
| Ref : Chemical Research in Toxicology , 5 :411 , 1992 |
| Abstract : |
| PubMedSearch : Lee_1992_Chem.Res.Toxicol_5_411 |
| PubMedID: 1504265 |
| Title : Metabolites of neostigmine and pyridostigmine do not contribute to antagonism of neuromuscular blockade in the dog - Hennis_1984_Anesthesiology_61_534 |
| Author(s) : Hennis PJ , Cronnelly R , Sharma M , Fisher DM , Miller RD |
| Ref : Anesthesiology , 61 :534 , 1984 |
| Abstract : |
| PubMedSearch : Hennis_1984_Anesthesiology_61_534 |
| PubMedID: 6149707 |