Propetamphos
General
Type : Organophosphate, Insecticide, Sulfur Compound
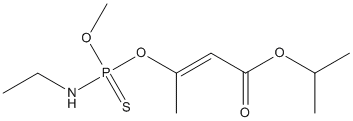
Chemical_Nomenclature : propan-2-yl (E)-3-[ethylamino(methoxy)phosphinothioyl]oxybut-2-enoate
Canonical SMILES : CCNP(=S)(OC)OC(=CC(=O)OC(C)C)C
InChI : InChI=1S\/C10H20NO4PS\/c1-6-11-16(17,13-5)15-9(4)7-10(12)14-8(2)3\/h7-8H,6H2,1-5H3,(H,11,17)\/b9-7+
InChIKey : BZNDWPRGXNILMS-VQHVLOKHSA-N
Other name(s) : 3-[(Ethylamino)methoxyphosphinothioyl]oxy]-2-butenoic acid,1-methylethyl ester || (E)-1-methylethyl 3-[{(ethylamino)methoxyphosphinothioly}oxy]-2-butenoate || Safrotin Zoecon || TSAR || Trade names for products containing propetamphos include Blotic, Safrotin and Seraphos. Commercial products include aerosols, emulsified concentrates, liquids, and powders
MW : 281.30
Formula : C10H20NO4PS
CAS_number : 31218-83-4
PubChem : 5372405
UniChem : BZNDWPRGXNILMS-VQHVLOKHSA-N
References (6)
| Title : Aging and spontaneous reactivation of human plasma cholinesterase activity after inhibition by organophosphorus pesticides - Mason_1993_Human.Exp.Toxicol_12_497 |
| Author(s) : Mason HJ , Waine E , Stevenson A , Wilson HK |
| Ref : Hum Exp Toxicol , 12 :497 , 1993 |
| Abstract : |
| PubMedSearch : Mason_1993_Human.Exp.Toxicol_12_497 |
| PubMedID: 7904465 |
| Title : Aging and spontaneous reactivation of human plasma cholinesterase activity after inhibition by organophosphorus pesticides - Mason_1993_Human.Exp.Toxicol_12_497 |
| Author(s) : Mason HJ , Waine E , Stevenson A , Wilson HK |
| Ref : Hum Exp Toxicol , 12 :497 , 1993 |
| Abstract : |
| PubMedSearch : Mason_1993_Human.Exp.Toxicol_12_497 |
| PubMedID: 7904465 |
| Title : Aging and spontaneous reactivation of human plasma cholinesterase activity after inhibition by organophosphorus pesticides - Mason_1993_Human.Exp.Toxicol_12_497 |
| Author(s) : Mason HJ , Waine E , Stevenson A , Wilson HK |
| Ref : Hum Exp Toxicol , 12 :497 , 1993 |
| Abstract : |
| PubMedSearch : Mason_1993_Human.Exp.Toxicol_12_497 |
| PubMedID: 7904465 |
| Title : Residual toxicity of wall-sprayed organophosphates, carbamates and pyrethroids to mosquito Culex pipiens molestus Forskal - Rettich_1980_J.Hyg.Epidemiol.Microbiol.Immunol_24_110 |
| Author(s) : Rettich F |
| Ref : J Hyg Epidemiol Microbiol Immunol , 24 :110 , 1980 |
| Abstract : |
| PubMedSearch : Rettich_1980_J.Hyg.Epidemiol.Microbiol.Immunol_24_110 |
| PubMedID: 7190586 |
| Title : Residual toxicity of wall-sprayed organophosphates, carbamates and pyrethroids to mosquito Culex pipiens molestus Forskal - Rettich_1980_J.Hyg.Epidemiol.Microbiol.Immunol_24_110 |
| Author(s) : Rettich F |
| Ref : J Hyg Epidemiol Microbiol Immunol , 24 :110 , 1980 |
| Abstract : |
| PubMedSearch : Rettich_1980_J.Hyg.Epidemiol.Microbiol.Immunol_24_110 |
| PubMedID: 7190586 |
| Title : Residual toxicity of wall-sprayed organophosphates, carbamates and pyrethroids to mosquito Culex pipiens molestus Forskal - Rettich_1980_J.Hyg.Epidemiol.Microbiol.Immunol_24_110 |
| Author(s) : Rettich F |
| Ref : J Hyg Epidemiol Microbiol Immunol , 24 :110 , 1980 |
| Abstract : |
| PubMedSearch : Rettich_1980_J.Hyg.Epidemiol.Microbiol.Immunol_24_110 |
| PubMedID: 7190586 |