TMPS
Impurity in malathion extremely toxic for mammals, structural isomer of TMP
General
Type : Organophosphate, Sulfur Compound
Chemical_Nomenclature : trimethoxy(sulfanylidene)-$l^{5}-phosphane
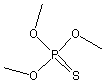
Canonical SMILES : COP(=S)(OC)OC
InChI : InChI=1S\/C3H9O3PS\/c1-4-7(8,5-2)6-3\/h1-3H3
InChIKey : XWSLYQXUTWUIKM-UHFFFAOYSA-N
Other name(s) : O,O,O-Trimethyl phosphorothioate || TMP=S || trimethoxy-sulfanylidene-phosphorane || Trimethyl thiophosphate || Trimethylthiophosphate || Trimethyl thionophosphate || O,O,O-Trimethylthiofosfat || Trimethoxyphosphine Sulfide || O,O,O-Trimethyl thiophosphate
MW : 156.142
Formula : C3H9O3PS
CAS_number : 152-18-1
PubChem : 9038
UniChem : XWSLYQXUTWUIKM-UHFFFAOYSA-N
Target
Families : TMPS ligand of proteins in family
ACHE
References (9)
| Title : Synthesis and 31P chemical shift identification of tripeptide active site models that represent human serum acetylcholinesterase covalently modified at serine by certain organophosphates - Thompson_1996_Chem.Res.Toxicol_9_1325 |
| Author(s) : Thompson CM , Suarez AI , Rodriguez OP |
| Ref : Chemical Research in Toxicology , 9 :1325 , 1996 |
| Abstract : |
| PubMedSearch : Thompson_1996_Chem.Res.Toxicol_9_1325 |
| PubMedID: 8951236 |
| Title : Synthesis and 31P chemical shift identification of tripeptide active site models that represent human serum acetylcholinesterase covalently modified at serine by certain organophosphates - Thompson_1996_Chem.Res.Toxicol_9_1325 |
| Author(s) : Thompson CM , Suarez AI , Rodriguez OP |
| Ref : Chemical Research in Toxicology , 9 :1325 , 1996 |
| Abstract : |
| PubMedSearch : Thompson_1996_Chem.Res.Toxicol_9_1325 |
| PubMedID: 8951236 |
| Title : Synthesis and 31P chemical shift identification of tripeptide active site models that represent human serum acetylcholinesterase covalently modified at serine by certain organophosphates - Thompson_1996_Chem.Res.Toxicol_9_1325 |
| Author(s) : Thompson CM , Suarez AI , Rodriguez OP |
| Ref : Chemical Research in Toxicology , 9 :1325 , 1996 |
| Abstract : |
| PubMedSearch : Thompson_1996_Chem.Res.Toxicol_9_1325 |
| PubMedID: 8951236 |
| Title : Mutagenic and alkylating activities of organophosphate impurities of commercial malathion - Imamura_1985_Mutat.Res_155_1 |
| Author(s) : Imamura T , Talcott RE |
| Ref : Mutat Res , 155 :1 , 1985 |
| Abstract : |
| PubMedSearch : Imamura_1985_Mutat.Res_155_1 |
| PubMedID: 3881662 |
| Title : Mutagenic and alkylating activities of organophosphate impurities of commercial malathion - Imamura_1985_Mutat.Res_155_1 |
| Author(s) : Imamura T , Talcott RE |
| Ref : Mutat Res , 155 :1 , 1985 |
| Abstract : |
| PubMedSearch : Imamura_1985_Mutat.Res_155_1 |
| PubMedID: 3881662 |
| Title : Mutagenic and alkylating activities of organophosphate impurities of commercial malathion - Imamura_1985_Mutat.Res_155_1 |
| Author(s) : Imamura T , Talcott RE |
| Ref : Mutat Res , 155 :1 , 1985 |
| Abstract : |
| PubMedSearch : Imamura_1985_Mutat.Res_155_1 |
| PubMedID: 3881662 |
| Title : Toxicological properties of trialkyl phosphorothioate and dialkyl alkyl- and arylphosphonothioate esters - Fukuto_1983_J.Environ.Sci.Health.B_18_89 |
| Author(s) : Fukuto TR |
| Ref : J Environ Sci Health B , 18 :89 , 1983 |
| Abstract : |
| PubMedSearch : Fukuto_1983_J.Environ.Sci.Health.B_18_89 |
| PubMedID: 6833715 |
| Title : Toxicological properties of trialkyl phosphorothioate and dialkyl alkyl- and arylphosphonothioate esters - Fukuto_1983_J.Environ.Sci.Health.B_18_89 |
| Author(s) : Fukuto TR |
| Ref : J Environ Sci Health B , 18 :89 , 1983 |
| Abstract : |
| PubMedSearch : Fukuto_1983_J.Environ.Sci.Health.B_18_89 |
| PubMedID: 6833715 |
| Title : Toxicological properties of trialkyl phosphorothioate and dialkyl alkyl- and arylphosphonothioate esters - Fukuto_1983_J.Environ.Sci.Health.B_18_89 |
| Author(s) : Fukuto TR |
| Ref : J Environ Sci Health B , 18 :89 , 1983 |
| Abstract : |
| PubMedSearch : Fukuto_1983_J.Environ.Sci.Health.B_18_89 |
| PubMedID: 6833715 |