Valilactone
General
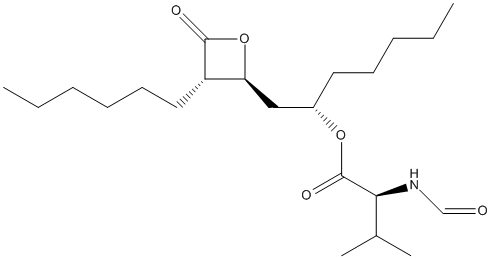
Chemical_Nomenclature : [(2S)-1-[(2S,3S)-3-hexyl-4-oxooxetan-2-yl]heptan-2-yl] (2S)-2-formamido-3-methylbutanoate
Canonical SMILES : CCCCCCC1C(OC1=O)CC(CCCCC)OC(=O)C(C(C)C)NC=O
InChI : InChI=1S\/C22H39NO5\/c1-5-7-9-11-13-18-19(28-21(18)25)14-17(12-10-8-6-2)27-22(26)20(16(3)4)23-15-24\/h15-20H,5-14H2,1-4H3,(H,23,24)\/t17-,18-,19-,20-\/m0\/s1
InChIKey : WWGVIIVMPMBQFV-MUGJNUQGSA-N
Other name(s) : (2s)-1-[(2s,3s)-3-hexyl-4-oxooxetan-2-yl]heptan-2-yl n-formyl-l-valinate || (2S)-1-[(2S,3S)-3-hexyl-4-oxooxetan-2-yl]heptan-2-yl (2S)-2-formamido-3-methylbutanoate || AC1L4ZA0 || AC1Q5X3S || CHEMBL492253
Target
Families : Valilactone ligand of proteins in family
Pancreatic_lipase
References (9)
| Title : Total synthesis and comparative analysis of orlistat, valilactone, and a transposed orlistat derivative: Inhibitors of fatty acid synthase - Ma_2006_Org.Lett_8_4497 |
| Author(s) : Ma G , Zancanella M , Oyola Y , Richardson RD , Smith JW , Romo D |
| Ref : Org Lett , 8 :4497 , 2006 |
| Abstract : |
| PubMedSearch : Ma_2006_Org.Lett_8_4497 |
| PubMedID: 16986934 |
| Title : An expeditious enantioselective total synthesis of valilactone - Wu_2006_J.Org.Chem_71_5748 |
| Author(s) : Wu Y , Sun YP |
| Ref : J Org Chem , 71 :5748 , 2006 |
| Abstract : |
| PubMedSearch : Wu_2006_J.Org.Chem_71_5748 |
| PubMedID: 16839158 |
| Title : Total synthesis and comparative analysis of orlistat, valilactone, and a transposed orlistat derivative: Inhibitors of fatty acid synthase - Ma_2006_Org.Lett_8_4497 |
| Author(s) : Ma G , Zancanella M , Oyola Y , Richardson RD , Smith JW , Romo D |
| Ref : Org Lett , 8 :4497 , 2006 |
| Abstract : |
| PubMedSearch : Ma_2006_Org.Lett_8_4497 |
| PubMedID: 16986934 |
| Title : An expeditious enantioselective total synthesis of valilactone - Wu_2006_J.Org.Chem_71_5748 |
| Author(s) : Wu Y , Sun YP |
| Ref : J Org Chem , 71 :5748 , 2006 |
| Abstract : |
| PubMedSearch : Wu_2006_J.Org.Chem_71_5748 |
| PubMedID: 16839158 |
| Title : Total synthesis and comparative analysis of orlistat, valilactone, and a transposed orlistat derivative: Inhibitors of fatty acid synthase - Ma_2006_Org.Lett_8_4497 |
| Author(s) : Ma G , Zancanella M , Oyola Y , Richardson RD , Smith JW , Romo D |
| Ref : Org Lett , 8 :4497 , 2006 |
| Abstract : |
| PubMedSearch : Ma_2006_Org.Lett_8_4497 |
| PubMedID: 16986934 |
| Title : An expeditious enantioselective total synthesis of valilactone - Wu_2006_J.Org.Chem_71_5748 |
| Author(s) : Wu Y , Sun YP |
| Ref : J Org Chem , 71 :5748 , 2006 |
| Abstract : |
| PubMedSearch : Wu_2006_J.Org.Chem_71_5748 |
| PubMedID: 16839158 |
| Title : Valilactone, an inhibitor of esterase, produced by actinomycetes - |
| Author(s) : Kitahara M , Asano M , Naganawa H , Maeda K , Hamada M , Aoyagi T , Umezawa H , Iitaka Y , Nakamura H |
| Ref : J Antibiot (Tokyo) , 40 :1647 , 1987 |
| PubMedID: 3693135 |
| Title : Valilactone, an inhibitor of esterase, produced by actinomycetes - |
| Author(s) : Kitahara M , Asano M , Naganawa H , Maeda K , Hamada M , Aoyagi T , Umezawa H , Iitaka Y , Nakamura H |
| Ref : J Antibiot (Tokyo) , 40 :1647 , 1987 |
| PubMedID: 3693135 |
| Title : Valilactone, an inhibitor of esterase, produced by actinomycetes - |
| Author(s) : Kitahara M , Asano M , Naganawa H , Maeda K , Hamada M , Aoyagi T , Umezawa H , Iitaka Y , Nakamura H |
| Ref : J Antibiot (Tokyo) , 40 :1647 , 1987 |
| PubMedID: 3693135 |