TerritremA
Mycotoxin purified from culture of fungus Aspergillus terreus 23-1 growing on rice
General
Type : Natural,Benzodioxo,Meroterpenoid
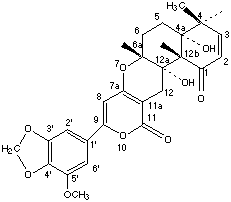
Chemical_Nomenclature : 4H,11H-Naphtho(2,1-b)pyrano(3,4-e)pyran-1,11(5H)-dione, 4a,6,6a,12,12a,12b-hexahydro-4a,12a-dihydroxy-9-(7-methoxy-1,3-benzodioxo-5-yl)-4,4,6a,12b-tetramethyl-, (4aR,6aR,12aS,12bS)-
Canonical SMILES : CC1(C=CC(=O)C2(C1(CCC3(C2(CC4=C(O3)C=C(OC4=O)C5=CC6=C(C(=C5)OC)OCO6)O)C)O)C)C
InChI : InChI=1S\/C28H30O9\/c1-24(2)7-6-21(29)26(4)27(24,31)9-8-25(3)28(26,32)13-16-18(37-25)12-17(36-23(16)30)15-10-19(33-5)22-20(11-15)34-14-35-22\/h6-7,10-12,31-32H,8-9,13-14H2,1-5H3
InChIKey : LCJHAHVVYAVVPA-UHFFFAOYSA-N
Other name(s) :
MW : 510.20
Formula : C28H30O9
CAS_number : 70407-19-1
PubChem : 115079
UniChem : LCJHAHVVYAVVPA-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
References (4)
| Title : NMR assignments of territrems A, B, and C and the structure of MB2, the major metabolite of territrem B by rat liver microsomal fraction - Lee_1992_J.Nat.Prod_55_251 |
| Author(s) : Lee SS , Peng FC , Chiou CM , Ling KH |
| Ref : Journal of Natural Products , 55 :251 , 1992 |
| Abstract : Lee_1992_J.Nat.Prod_55_251 |
| ESTHER : Lee_1992_J.Nat.Prod_55_251 |
| PubMedSearch : Lee_1992_J.Nat.Prod_55_251 |
| PubMedID: 1624944 |
| Title : Biotransformation of territrems by S9 fraction from rat liver - Ling_1991_Drug.Metab.Dispos_19_587 |
| Author(s) : Ling KH , Chiou CM , Tseng YL |
| Ref : Drug Metabolism & Disposition: The Biological Fate of Chemicals , 19 :587 , 1991 |
| Abstract : Ling_1991_Drug.Metab.Dispos_19_587 |
| ESTHER : Ling_1991_Drug.Metab.Dispos_19_587 |
| PubMedSearch : Ling_1991_Drug.Metab.Dispos_19_587 |
| PubMedID: 1680623 |
| Title : Solvent systems for improved isolation and separation of territrems A and B - Ling_1982_Appl.Environ.Microbiol_44_860 |
| Author(s) : Ling KH , Yang CK , Kuo CA , Kuo MD |
| Ref : Applied Environmental Microbiology , 44 :860 , 1982 |
| Abstract : Ling_1982_Appl.Environ.Microbiol_44_860 |
| ESTHER : Ling_1982_Appl.Environ.Microbiol_44_860 |
| PubMedSearch : Ling_1982_Appl.Environ.Microbiol_44_860 |
| PubMedID: 7149718 |