Tolserine
Derivative of Physostigmine (Eserine)
General
Type : Derivative of physostigmine-eserine,Carbamate,Indole,NTE inhibitor,Not A\/B H target
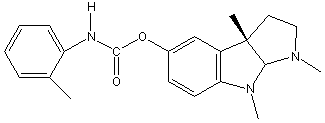
Chemical_Nomenclature : [(3aR,8bS)-3,4,8b-trimethyl-2,3a-dihydro-1H-pyrrolo[2,3-b]indol-7-yl] N-(2-methylphenyl)carbamate
Canonical SMILES : CC1=CC=CC=C1NC(=O)OC2=CC3=C(C=C2)N(C4C3(CCN4C)C)C
InChI : InChI=1S\/C21H25N3O2\/c1-14-7-5-6-8-17(14)22-20(25)26-15-9-10-18-16(13-15)21(2)11-12-23(3)19(21)24(18)4\/h5-10,13,19H,11-12H2,1-4H3,(H,22,25)\/t19-,21+\/m1\/s1
InChIKey : JGAGHIIOCADQOV-CTNGQTDRSA-N
Other name(s) : (-)-2'-methyl-phenylcarbamoyl eseroline,CHEMBL130458,SCHEMBL8920175,DNC006354
MW : 351.44
Formula : C21H25N3O2
CAS_number :
PubChem : 9928397
UniChem : JGAGHIIOCADQOV-CTNGQTDRSA-N
IUPHAR :
Wikipedia :
Target
References (2)
| Title : Kinetics of human acetylcholinesterase inhibition by the novel experimental Alzheimer therapeutic agent, tolserine - Kamal_2000_Biochem.Pharmacol_60_561 |
| Author(s) : Kamal MA , Greig NH , Alhomida AS , Al-Jafari AA |
| Ref : Biochemical Pharmacology , 60 :561 , 2000 |
| Abstract : Kamal_2000_Biochem.Pharmacol_60_561 |
| ESTHER : Kamal_2000_Biochem.Pharmacol_60_561 |
| PubMedSearch : Kamal_2000_Biochem.Pharmacol_60_561 |
| PubMedID: 10874131 |
| Title : Synthesis of novel phenserine-based-selective inhibitors of butyrylcholinesterase for Alzheimer's disease - Yu_1999_J.Med.Chem_42_1855 |
| Author(s) : Yu Q , Holloway HW , Utsuki T , Brossi A , Greig NH |
| Ref : Journal of Medicinal Chemistry , 42 :1855 , 1999 |
| Abstract : Yu_1999_J.Med.Chem_42_1855 |
| ESTHER : Yu_1999_J.Med.Chem_42_1855 |
| PubMedSearch : Yu_1999_J.Med.Chem_42_1855 |
| PubMedID: 10346939 |