Anordrin
post-coital contraceptive drug that is on the market in China for more than 30 years. CES1 and CES2 generate the main active metabolite, anordiol
General
Type : Pro-Drug, Drug, Propionate, Alkyne
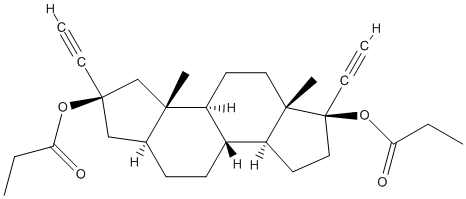
Chemical_Nomenclature : [(2R,3aS,3bS,5aS,6R,8aS,8bR,10aS)-2,6-diethynyl-3a,5a-dimethyl-2-propanoyloxy-1,3,3b,4,5,7,8,8a,8b,9,10,10a-dodecahydroindeno[5,4-e]inden-6-yl] propanoate
Canonical SMILES : CCC(=O)OC1(CCC2C1(CCC3C2CCC4C3(CC(C4)(C#C)OC(=O)CC)C)C)C#C
InChI : InChI=1S\/C28H38O4\/c1-7-23(29)31-27(9-3)17-19-11-12-20-21(25(19,5)18-27)13-15-26(6)22(20)14-16-28(26,10-4)32-24(30)8-2\/h3-4,19-22H,7-8,11-18H2,1-2,5-6H3\/t19-,20+,21-,22-,25-,26-,27+,28-\/m0\/s1
InChIKey : NOECSYBNZHIVHW-LKADTRSGSA-N
Other name(s) : alpha-Anordrin || AF-53 || 6R25F2A061 || SCHEMBL18906509 || 2alpha, 17alpha-diethynyl-A-nor-5alpha-androstane-2beta, 17beta-diol dipropionate
MW : 438.6
Formula : C28H38O4
CAS_number : 56470-64-5
PubChem : 6917889
UniChem : NOECSYBNZHIVHW-LKADTRSGSA-N
Target
Structures : No structure
Families : Carb_B_Chordata
References (3)
| Title : Predominant contributions of carboxylesterase 1 and 2 in hydrolysis of anordrin in humans - Jiang_2017_Xenobiotica__1 |
| Author(s) : Jiang J , Chen X , Zhong D |
| Ref : Xenobiotica , :1 , 2017 |
| Abstract : |
| PubMedSearch : Jiang_2017_Xenobiotica__1 |
| PubMedID: 28532270 |
| Gene_locus related to this paper: human-CES1 |
| Title : Predominant contributions of carboxylesterase 1 and 2 in hydrolysis of anordrin in humans - Jiang_2017_Xenobiotica__1 |
| Author(s) : Jiang J , Chen X , Zhong D |
| Ref : Xenobiotica , :1 , 2017 |
| Abstract : |
| PubMedSearch : Jiang_2017_Xenobiotica__1 |
| PubMedID: 28532270 |
| Gene_locus related to this paper: human-CES1 |
| Title : Predominant contributions of carboxylesterase 1 and 2 in hydrolysis of anordrin in humans - Jiang_2017_Xenobiotica__1 |
| Author(s) : Jiang J , Chen X , Zhong D |
| Ref : Xenobiotica , :1 , 2017 |
| Abstract : |
| PubMedSearch : Jiang_2017_Xenobiotica__1 |
| PubMedID: 28532270 |
| Gene_locus related to this paper: human-CES1 |