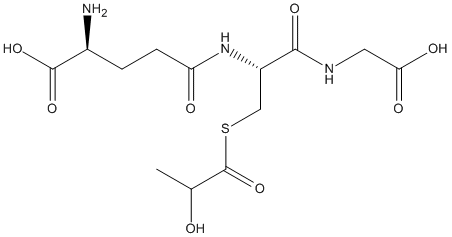
S-Lactoylglutathione
General
Type : Peptide
Chemical_Nomenclature : (2S)-2-amino-5-[[(2R)-1-(carboxymethylamino)-3-(2-hydroxypropanoylsulfanyl)-1-oxopropan-2-yl]amino]-5-oxopentanoic acid
Canonical SMILES : CC(C(=O)SCC(C(=O)NCC(=O)O)NC(=O)CCC(C(=O)O)N)O
InChI : InChI=1S\/C13H21N3O8S\/c1-6(17)13(24)25-5-8(11(21)15-4-10(19)20)16-9(18)3-2-7(14)12(22)23\/h6-8,17H,2-5,14H2,1H3,(H,15,21)(H,16,18)(H,19,20)(H,22,23)\/t6?,7-,8-\/m0\/s1
InChIKey : VDYDCVUWILIYQF-ALKRTJFJSA-N
Other name(s) : S-Lactylglutathione || Glutathione, S-lactate (6CI,7CI) || S-D-lactoylglutathione || Glycine, N-(N-L-gamma-glutamyl-S-(2-hydroxy-1-oxopropyl)-L-cysteinyl)- || Glycine, l-|A-glutamyl-s-(2-hydroxy-1-oxopropyl)-l-cysteinyl-
MW : 379.38
Formula : C13H21N3O8S
CAS_number :
PubChem : 119450
UniChem : VDYDCVUWILIYQF-ALKRTJFJSA-N
Target
Structures : No structure
Families : A85-EsteraseD-FGH
References (3)
| Title : A role for His-160 in peroxide inhibition of S. cerevisiae S-formylglutathione hydrolase: evidence for an oxidation sensitive motif - Legler_2012_Arch.Biochem.Biophys_528_7 |
| Author(s) : Legler PM , Leary DH , Hervey WJt , Millard CB |
| Ref : Archives of Biochemistry & Biophysics , 528 :7 , 2012 |
| Abstract : |
| PubMedSearch : Legler_2012_Arch.Biochem.Biophys_528_7 |
| PubMedID: 22906720 |
| Gene_locus related to this paper: yeast-yjg8 |
| Title : A role for His-160 in peroxide inhibition of S. cerevisiae S-formylglutathione hydrolase: evidence for an oxidation sensitive motif - Legler_2012_Arch.Biochem.Biophys_528_7 |
| Author(s) : Legler PM , Leary DH , Hervey WJt , Millard CB |
| Ref : Archives of Biochemistry & Biophysics , 528 :7 , 2012 |
| Abstract : |
| PubMedSearch : Legler_2012_Arch.Biochem.Biophys_528_7 |
| PubMedID: 22906720 |
| Gene_locus related to this paper: yeast-yjg8 |
| Title : A role for His-160 in peroxide inhibition of S. cerevisiae S-formylglutathione hydrolase: evidence for an oxidation sensitive motif - Legler_2012_Arch.Biochem.Biophys_528_7 |
| Author(s) : Legler PM , Leary DH , Hervey WJt , Millard CB |
| Ref : Archives of Biochemistry & Biophysics , 528 :7 , 2012 |
| Abstract : |
| PubMedSearch : Legler_2012_Arch.Biochem.Biophys_528_7 |
| PubMedID: 22906720 |
| Gene_locus related to this paper: yeast-yjg8 |