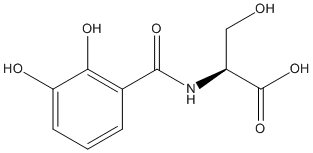
2,3-Dihydroxybenzoylserine
General
Type :
Chemical_Nomenclature : (2S)-2-[(2,3-dihydroxybenzoyl)amino]-3-hydroxypropanoic acid
Canonical SMILES : C1=CC(=C(C(=C1)O)O)C(=O)NC(CO)C(=O)O
InChI : InChI=1S\/C10H11NO6\/c12-4-6(10(16)17)11-9(15)5-2-1-3-7(13)8(5)14\/h1-3,6,12-14H,4H2,(H,11,15)(H,16,17)\/t6-\/m0\/s1
InChIKey : VDTYHTVHFIIEIL-LURJTMIESA-N
Other name(s) : 2,3-di-hydroxy-N-benzoyl-serine, N-(2,3-dihydroxybenzoyl)-L-serine, 2,3,-DIHYDROXYBENZOYLSERINE, 2,3-Dihydroxy-N-benzoyl-L-serine, L-Serine, N-(2,3-dihydroxybenzoyl)-
Target
Families : A85-IroE-IroD-Fes-Yiel
References
No reference