CHEMBL3104293
IC50 ACHE 45.5 +/- 4.4 microM BCHE 0.903 +/- 0.003 micro M This is the most active compound against BCHE in Kratky et al. 2014. Many more compounds of this class were tested by the same group but were not more active compounds (Imramovsky et al. 2012) and Phosphorothioates analogues were tested also for antimycobacterial activity Vinsova et al. 2014
General
Type : Organophosphate, Carboxamide
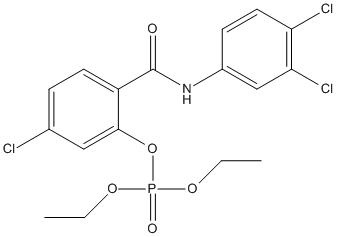
Chemical_Nomenclature : [5-chloro-2-[(3,4-dichlorophenyl)carbamoyl]phenyl] diethyl phosphate
Canonical SMILES : CCOP(=O)(OCC)OC1=C(C=CC(=C1)Cl)C(=O)NC2=CC(=C(C=C2)Cl)Cl
InChI : InChI=1S\/C17H17Cl3NO5P\/c1-3-24-27(23,25-4-2)26-16-9-11(18)5-7-13(16)17(22)21-12-6-8-14(19)15(20)10-12\/h5-10H,3-4H2,1-2H3,(H,21,22)
InChIKey : VAVYJVCTFBRYSO-UHFFFAOYSA-N
Other name(s) : Compound 25 || BDBM150745 || [5-chloro-2-[(3,4-dichlorophenyl)carbamoyl]phenyl] diethyl phosphate || 5-chloro-2-[(3,4-dichlorophenyl)carbamoyl]phenyl diethyl phosphate (25)
MW : 452.6
Formula : C17H17Cl3NO5P
CAS_number :
PubChem : 73334558
UniChem : VAVYJVCTFBRYSO-UHFFFAOYSA-N
Target
Families : CHEMBL3104293 ligand of proteins in family
BCHE
References (3)
| Title : Salicylanilide diethyl phosphates as cholinesterases inhibitors - Kratky_2014_Bioorg.Chem_58C_48 |
| Author(s) : Kratky M , Stepankova S , Vorcakova K , Vinsova J |
| Ref : Bioorg Chem , 58C :48 , 2014 |
| Abstract : |
| PubMedSearch : Kratky_2014_Bioorg.Chem_58C_48 |
| PubMedID: 25462625 |
| Title : Diethyl 2-(Phenylcarbamoyl)phenyl Phosphorothioates: Synthesis, Antimycobacterial Activity and Cholinesterase Inhibition - Vinsova_2014_Molecules_19_7152 |
| Author(s) : Vinsova J , Kratky M , Komloova M , Dadapeer E , Stepankova S , Vorcakova K , Stolarikova J |
| Ref : Molecules , 19 :7152 , 2014 |
| Abstract : |
| PubMedSearch : Vinsova_2014_Molecules_19_7152 |
| PubMedID: 24886941 |
| Title : Acetylcholinesterase-inhibiting activity of salicylanilide N-alkylcarbamates and their molecular docking - Imramovsky_2012_Molecules_17_10142 |
| Author(s) : Imramovsky A , Stepankova S , Vanco J , Pauk K , Monreal-Ferriz J , Vinsova J , Jampilek J |
| Ref : Molecules , 17 :10142 , 2012 |
| Abstract : |
| PubMedSearch : Imramovsky_2012_Molecules_17_10142 |
| PubMedID: 22922284 |