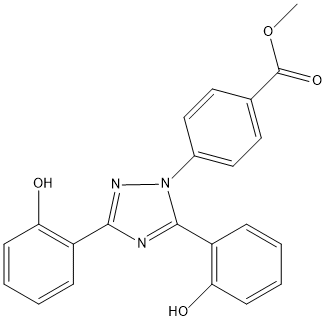
Deferasirox-Methyl-Ester
Deferasirox-substituted hydrazide compound 10 (IC50 +/- SEM) of 6.24 +/- 0.16 0.45 +/- 0.02 33.59 +/- 3.09 19.55 +/- 1.94 1.12 +/- 0.14 microM against AChE, MAO-A, MAO-B and COX-2
General
Type : Monoamine-oxidase-inhibitor, Multitarget, Triazol, Benzoate
Chemical_Nomenclature : methyl 4-[3,5-bis(2-hydroxyphenyl)-1,2,4-triazol-1-yl]benzoate
Canonical SMILES : COC(=O)C1=CC=C(C=C1)N2C(=NC(=N2)C3=CC=CC=C3O)C4=CC=CC=C4O
InChI : InChI=1S\/C22H17N3O4\/c1-29-22(28)14-10-12-15(13-11-14)25-21(17-7-3-5-9-19(17)27)23-20(24-25)16-6-2-4-8-18(16)26\/h2-13,26-27H,1H3
InChIKey : SQUCUUVFSUCLCC-UHFFFAOYSA-N
Other name(s) : CHEMBL5178246 || Benzoic acid, 4-[3,5-bis(2-hydroxyphenyl)-1H-1,2,4-triazol-1-yl]-, methyl ester || Deferasirox Methyl Ester || Compound 10 || SCHEMBL8242279
MW : 387.4
Formula : C22H17N3O4
CAS_number : 1266741-05-2
PubChem : 136119657
UniChem : SQUCUUVFSUCLCC-UHFFFAOYSA-N
Target
References (1)
| Title : Exploration of novel triazolo-thiadiazine hybrids of deferasirox as multi-target-directed anti-neuroinflammatory agents with structure-activity relationship (SAR): a new treatment opportunity for Alzheimer's disease - Shakir_2025_RSC.Adv_15_101 |
| Author(s) : Shakir SA , Rashid U , Marryum , Fatima N , Ejaz SA , Fayyaz A , Ullah MZ , Saeed A , Khan A , Al Harrasi A , Mumtaz A |
| Ref : RSC Adv , 15 :101 , 2025 |
| Abstract : |
| PubMedSearch : Shakir_2025_RSC.Adv_15_101 |
| PubMedID: 39758929 |